正在加载图片...
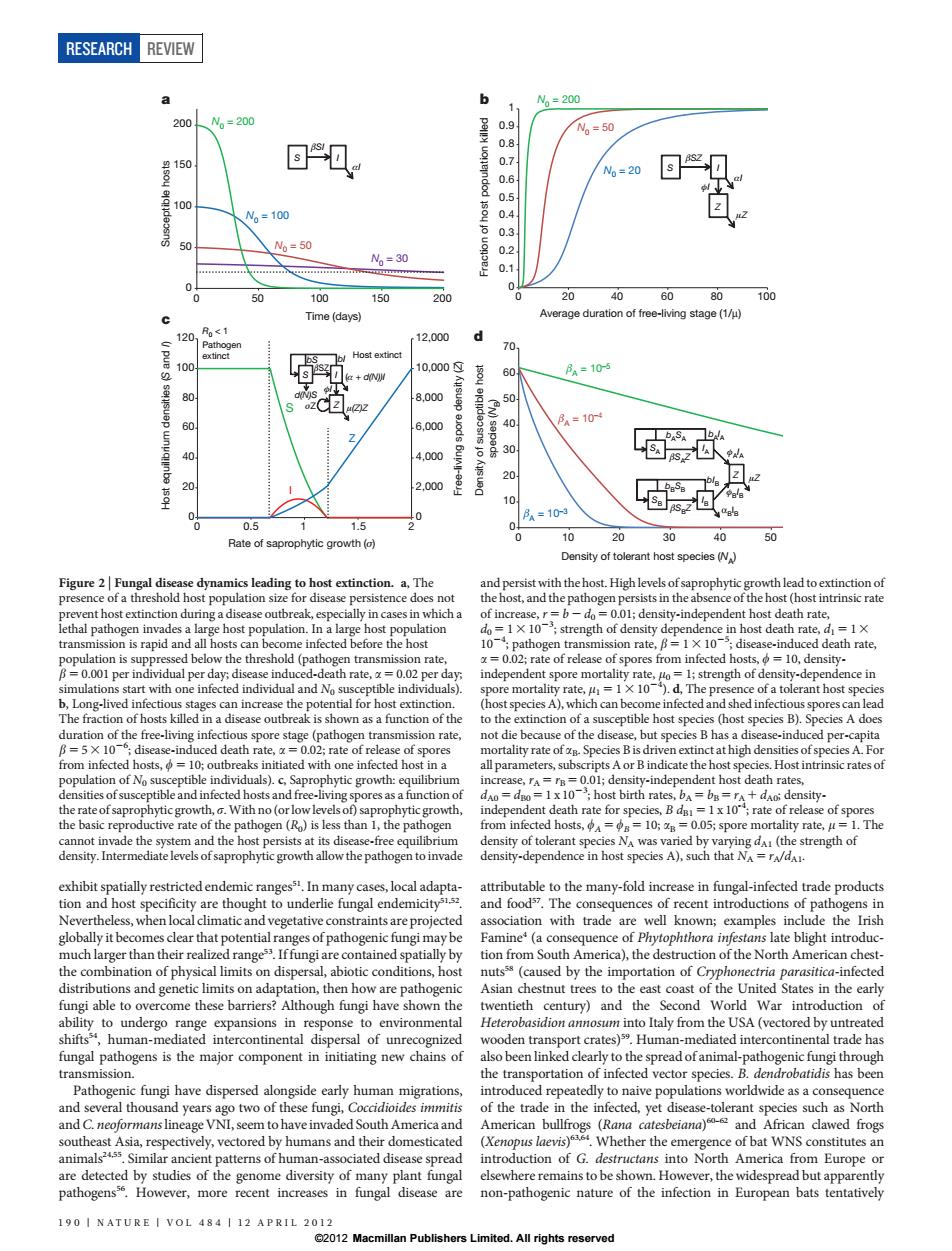
RESEARCH REVIEW b N。=200 1 200 N。=200 0.9 Ng=50 0.8 0.7 0.6 N%=20 0.5 N。=100 0.4 0.3 50 N6=50 N。=30 0.1 0 0 0 100 150 200 20 40 60 80 100 Time(days) Average duration of free-living stage(1/u) 120 Ro<1 12,000 d Pathogen 70 extinct Host extinct 100 10,000 60. 月a=105 8.000 50 22 60 6,000 40 Pa=10 40 4,000 % 20 2,000 10 0 .0 Pa=10-3 ag 0.5 1.5 2 0 20 30 50 Rate of saprophytic growth (o) Density of tolerant host species (N) Figure 2 Fungal disease dynamics leading to host extinction.a,The and persist with the host.High levels of saprophytic growth lead to extinction of presence of a threshold host population size for disease persistence does not the host,and the pathogen persists in the absence of the host(host intrinsic rate prevent host extinction during a disease outbreak,especially in cases in which a of increase,r=b-do 0.01;density-independent host death rate, lethal pathogen invades a large host population.In a large host population do=1 X 10;strength of density dependence in host death rate,d=1x transmission is rapid and all hosts can become infected before the host 10;pathogen transmission rate,=1 X 10;disease-induced death rate, population is suppressed below the threshold (pathogen transmission rate, =0.02;rate of release of spores from infected hosts,=10,density- B=0.001 per individual per day;disease induced-death rate,=0.02 per day independent spore mortality rate,o=1;strength of density-dependence in simulations start with one infected individual and No susceptible individuals). spore mortality rate,u=1 X 10).d,The presence of a tolerant host species b,Long-lived infectious stages can increase the potential for host extinction. (host species A),which can become infected and shed infectious spores can lead The fraction of hosts killed in a disease outbreak is shown as a function of the to the extinction of a susceptible host species (host species B).Species A does duration of the free-living infectious spore stage(pathogen transmission rate, not die because of the disease,but species B has a disease-induced per-capita B=5X 10;disease-induced death rate,=0.02;rate of release of spores mortality rate of ap.Species Bis driven extinct at high densities of species A.For from infected hosts,10;outbreaks initiated with one infected host in a all parameters,subscripts A or B indicate the host species.Host intrinsic rates of population of No susceptible individuals).c,Saprophytic growth:equilibrium increase,rA=rB=0.01;density-independent host death rates, densities of susceptible and infected hosts and free-living spores as a function of dAo=dBo=1 x 10;host birth rates,bA=bB=ra+dAo:density- the rate of saprophytic growth,o.With no (or low levels of)saprophytic growth, independent death rate for species,Bd=1x10;rate of release of spores the basic reproductive rate of the pathogen(Ro)is less than 1,the pathogen from infected hosts,==10;=0.05;spore mortality rate,=1.The cannot invade the system and the host persists at its disease-free equilibrium density of tolerant species NA was varied by varying dA(the strength of density.Intermediate levels of saprophytic growth allow the pathogen to invade density-dependence in host species A),such that NA=rA/dA. exhibit spatially restricted endemic rangess.In many cases,local adapta- attributable to the many-fold increase in fungal-infected trade products tion and host specificity are thought to underlie fungal endemicitys5 and foods7.The consequences of recent introductions of pathogens in Nevertheless,when local climatic and vegetative constraints are projected association with trade are well known;examples include the Irish globally it becomes clear that potential ranges of pathogenic fungi may be Famine(a consequence of Phytophthora infestans late blight introduc- much larger than their realized range Iffungi are contained spatially by tion from South America),the destruction of the North American chest- the combination of physical limits on dispersal,abiotic conditions,host nutsss(caused by the importation of Cryphonectria parasitica-infected distributions and genetic limits on adaptation,then how are pathogenic Asian chestnut trees to the east coast of the United States in the early fungi able to overcome these barriers?Although fungi have shown the twentieth century)and the Second World War introduction of ability to undergo range expansions in response to environmental Heterobasidion annosum into Italy from the USA(vectored by untreated shifts,human-mediated intercontinental dispersal of unrecognized wooden transport crates)s.Human-mediated intercontinental trade has fungal pathogens is the major component in initiating new chains of also been linked clearly to the spread of animal-pathogenic fungi through transmission. the transportation of infected vector species.B.dendrobatidis has been Pathogenic fungi have dispersed alongside early human migrations, introduced repeatedly to naive populations worldwide as a consequence and several thousand years ago two of these fungi,Coccidioides immitis of the trade in the infected,yet disease-tolerant species such as North and C.neoformans lineage VNI,seem to have invaded South America and American bullfrogs (Rana catesbeiana)and African clawed frogs southeast Asia,respectively,vectored by humans and their domesticated (Xenopus laevis).Whether the emergence of bat WNS constitutes an animals455 Similar ancient patterns of human-associated disease spread introduction of G.destructans into North America from Europe or are detected by studies of the genome diversity of many plant fungal elsewhere remains to be shown.However,the widespread but apparently pathogensse.However,more recent increases in fungal disease are non-pathogenic nature of the infection in European bats tentatively 190 NATURE VOL 484 12 APRIL 2012 2012 Macmillan Publishers Limited.All rights reservedexhibit spatially restricted endemic ranges51. In many cases, local adaptation and host specificity are thought to underlie fungal endemicity51,52. Nevertheless, when local climatic and vegetative constraints are projected globally it becomes clear that potential ranges of pathogenic fungi may be much larger than their realized range53. If fungi are contained spatially by the combination of physical limits on dispersal, abiotic conditions, host distributions and genetic limits on adaptation, then how are pathogenic fungi able to overcome these barriers? Although fungi have shown the ability to undergo range expansions in response to environmental shifts54, human-mediated intercontinental dispersal of unrecognized fungal pathogens is the major component in initiating new chains of transmission. Pathogenic fungi have dispersed alongside early human migrations, and several thousand years ago two of these fungi, Coccidioides immitis and C. neoformanslineage VNI, seem to have invaded South America and southeast Asia, respectively, vectored by humans and their domesticated animals24,55. Similar ancient patterns of human-associated disease spread are detected by studies of the genome diversity of many plant fungal pathogens56. However, more recent increases in fungal disease are attributable to the many-fold increase in fungal-infected trade products and food57. The consequences of recent introductions of pathogens in association with trade are well known; examples include the Irish Famine4 (a consequence of Phytophthora infestans late blight introduction from South America), the destruction of the North American chestnuts58 (caused by the importation of Cryphonectria parasitica-infected Asian chestnut trees to the east coast of the United States in the early twentieth century) and the Second World War introduction of Heterobasidion annosum into Italy from the USA (vectored by untreated wooden transport crates)59. Human-mediated intercontinental trade has also been linked clearly to the spread of animal-pathogenic fungi through the transportation of infected vector species. B. dendrobatidis has been introduced repeatedly to naive populations worldwide as a consequence of the trade in the infected, yet disease-tolerant species such as North American bullfrogs (Rana catesbeiana) 60–62 and African clawed frogs (Xenopus laevis) 63,64. Whether the emergence of bat WNS constitutes an introduction of G. destructans into North America from Europe or elsewhere remains to be shown. However, the widespread but apparently non-pathogenic nature of the infection in European bats tentatively 0 50 100 150 200 0 50 100 150 200 Susceptible hosts Time (days) N0 = 100 N0 = 50 N0 = 200 N0 = 30 0 10 20 30 40 50 60 70 0 10 20 30 40 50 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1 0 20 40 60 80 100 Fraction of host population killed Average duration of free-living stage (1/μ) 0 2,000 4,000 6,000 8,000 10,000 12,000 0 20 40 60 80 100 120 0 0.5 1 1.5 2 Free-living spore density (Z) Host equilibrium densities (S and I) Rate of saprophytic growth (σ) R0 < 1 Pathogen extinct Host extinct S I Z S I βSI S I βSZ αI Z φI μZ S I βSZ (α + d(N))I Z μ(Z)Z bS bI d(N)S SA I A bAI A SB Z μZ a b c d αl N0 = 200 N0 = 50 N0 = 20 Density of susceptible host species (NB) Density of tolerant host species (NA) φAI A φBI B αBI B I B βSBZ bBSB bIB βSAZ bASA βA = 10–5 βA = 10–4 βA = 10–3 φI σZ Figure 2 | Fungal disease dynamics leading to host extinction. a, The presence of a threshold host population size for disease persistence does not prevent host extinction during a disease outbreak, especially in cases in which a lethal pathogen invades a large host population. In a large host population transmission is rapid and all hosts can become infected before the host population is suppressed below the threshold (pathogen transmission rate, b 5 0.001 per individual per day; disease induced-death rate, a 5 0.02 per day; simulations start with one infected individual and N0 susceptible individuals). b, Long-lived infectious stages can increase the potential for host extinction. The fraction of hosts killed in a disease outbreak is shown as a function of the duration of the free-living infectious spore stage (pathogen transmission rate, b 5 5 3 1026 ; disease-induced death rate, a 5 0.02; rate of release of spores from infected hosts, w 5 10; outbreaks initiated with one infected host in a population of N0 susceptible individuals). c, Saprophytic growth: equilibrium densities of susceptible and infected hosts and free-living spores as a function of the rate of saprophytic growth, s.With no (or low levels of) saprophytic growth, the basic reproductive rate of the pathogen (R0) is less than 1, the pathogen cannot invade the system and the host persists at its disease-free equilibrium density. Intermediate levels of saprophytic growth allow the pathogen to invade and persist with the host. High levels of saprophytic growth lead to extinction of the host, and the pathogen persists in the absence of the host (host intrinsic rate of increase, r 5 b 2 d0 5 0.01; density-independent host death rate, d0 5 1 3 1023 ; strength of density dependence in host death rate, d1 5 1 3 1024 ; pathogen transmission rate, b 5 1 3 1025 ; disease-induced death rate, a 5 0.02; rate of release of spores from infected hosts, w 5 10, densityindependent spore mortality rate, m0 5 1; strength of density-dependence in spore mortality rate, m1 5 1 3 1024 ). d, The presence of a tolerant host species (host species A), which can become infected and shed infectious spores can lead to the extinction of a susceptible host species (host species B). Species A does not die because of the disease, but species B has a disease-induced per-capita mortality rate of aB. Species B is driven extinct at high densities of species A. For all parameters, subscripts A or B indicate the host species. Host intrinsic rates of increase, rA5 rB5 0.01; density-independent host death rates, dA0 5 dB05 1 x 1023 ; host birth rates, bA5 bB5 rA1 dA0; densityindependent death rate for species, B dB1 5 1 x 10-4; rate of release of spores from infected hosts, wA 5 wB 5 10; aB 5 0.05; spore mortality rate, m 5 1. The density of tolerant species NA was varied by varying dA1 (the strength of density-dependence in host species A), such that NA 5 rA/dA1. RESEARCH REVIEW 190 | NATURE | VOL 484 | 12 APRIL 2012 ©2012 Macmillan Publishers Limited. All rights reserved