正在加载图片...
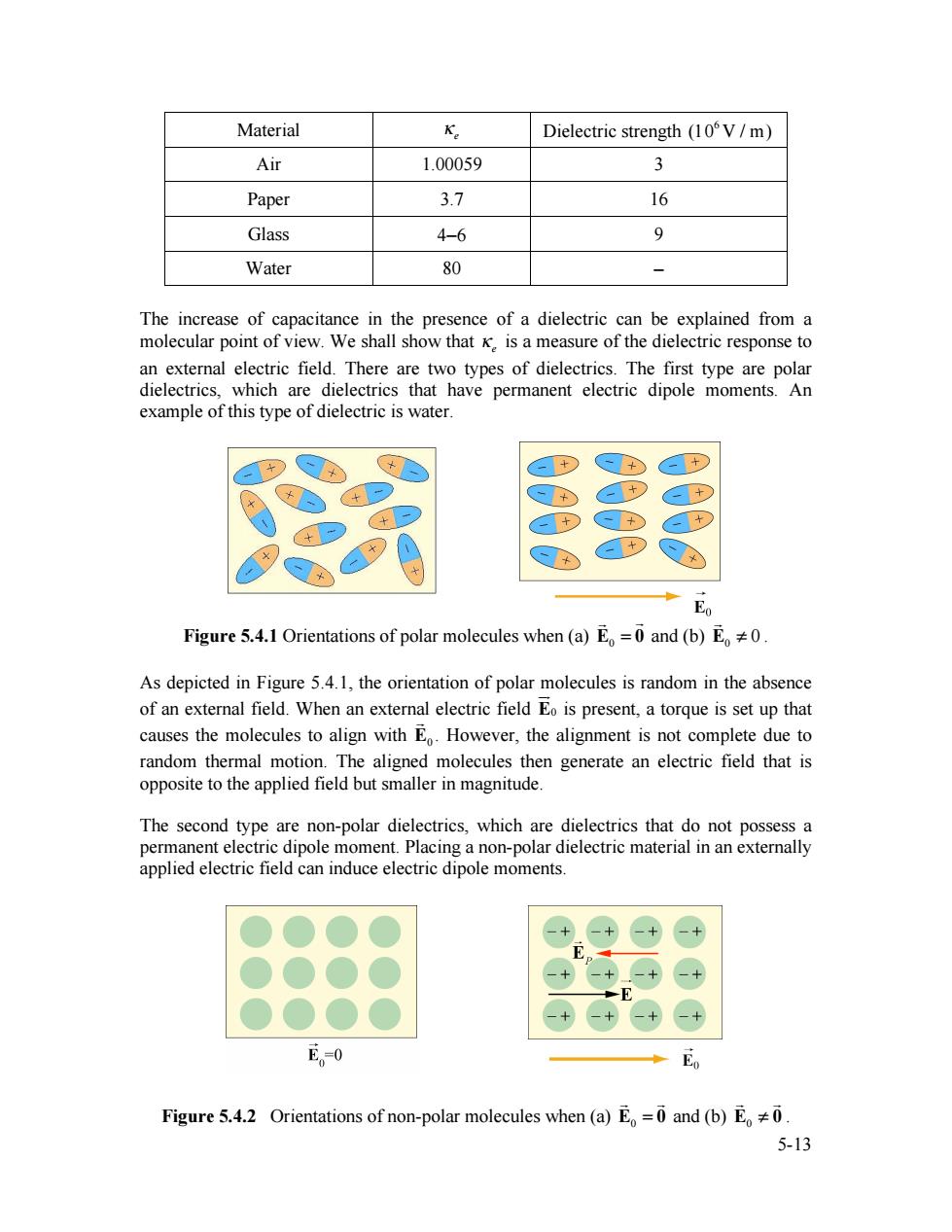
Material K Dielectric strength (10V/m) Air 1.00059 3 Paper 3.7 16 Glass 4-6 9 Water 80 - The increase of capacitance in the presence of a dielectric can be explained from a molecular point of view.We shall show that K.is a measure of the dielectric response to an external electric field.There are two types of dielectrics.The first type are polar dielectrics,which are dielectrics that have permanent electric dipole moments.An example of this type of dielectric is water. Eo Figure 5.4.1 Orientations of polar molecules when (a)E=0 and (b)E0. As depicted in Figure 5.4.1,the orientation of polar molecules is random in the absence of an external field.When an external electric field Eo is present,a torque is set up that causes the molecules to align with E.However,the alignment is not complete due to random thermal motion.The aligned molecules then generate an electric field that is opposite to the applied field but smaller in magnitude. The second type are non-polar dielectrics,which are dielectrics that do not possess a permanent electric dipole moment.Placing a non-polar dielectric material in an externally applied electric field can induce electric dipole moments. E。0 E Figure 5.4.2 Orientations of non-polar molecules when (a)E=0 and (b)E0 5-135-13 Material !e Dielectric strength (106 V / m) Air 1.00059 3 Paper 3.7 16 Glass 4−6 9 Water 80 − The increase of capacitance in the presence of a dielectric can be explained from a molecular point of view. We shall show that !e is a measure of the dielectric response to an external electric field. There are two types of dielectrics. The first type are polar dielectrics, which are dielectrics that have permanent electric dipole moments. An example of this type of dielectric is water. Figure 5.4.1 Orientations of polar molecules when (a) E0 = 0 ! ! and (b) 0 E ! 0 ! . As depicted in Figure 5.4.1, the orientation of polar molecules is random in the absence of an external field. When an external electric field E0 !" is present, a torque is set up that causes the molecules to align with E0 ! . However, the alignment is not complete due to random thermal motion. The aligned molecules then generate an electric field that is opposite to the applied field but smaller in magnitude. The second type are non-polar dielectrics, which are dielectrics that do not possess a permanent electric dipole moment. Placing a non-polar dielectric material in an externally applied electric field can induce electric dipole moments. Figure 5.4.2 Orientations of non-polar molecules when (a) E0 = 0 ! ! and (b) E0 ! 0 ! !