正在加载图片...
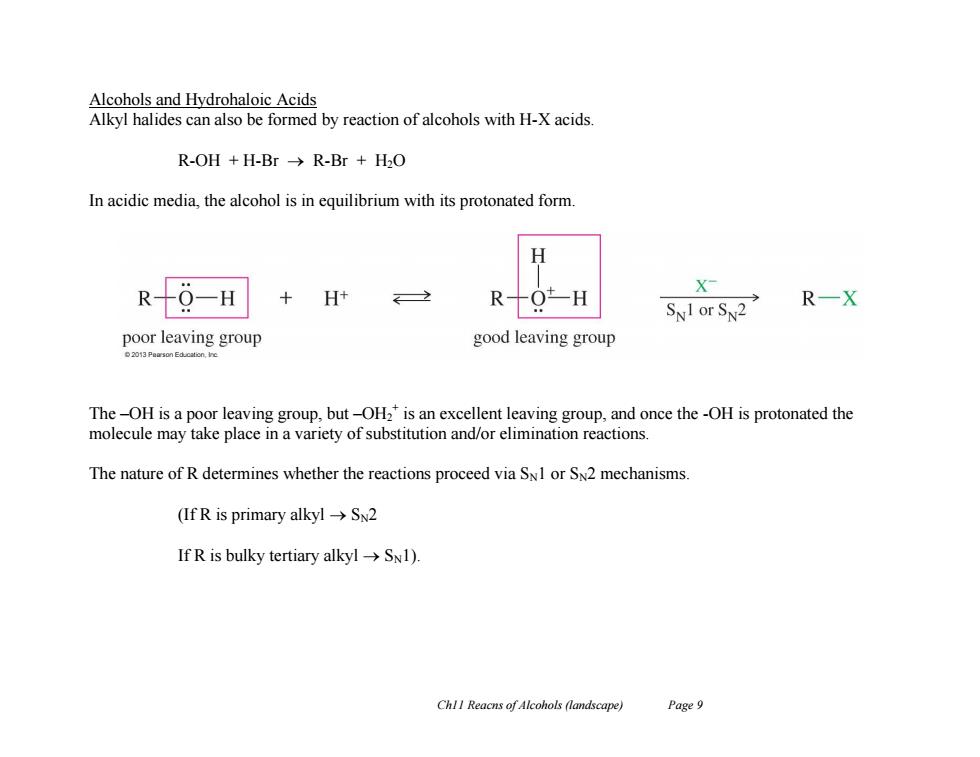
Alcohols and Hydrohaloic Acids Alkyl halides can also be formed by reaction of alcohols with H-X acids. R-OH+H-Br→R-Br+H2O In acidic media,the alcohol is in equilibrium with its protonated form. H R-9-H X H+ R十O-H SNI or SN2 R一X poor leaving group good leaving group 2013 Puarson Educalcn.ie The-OH is a poor leaving group,but-OH2 is an excellent leaving group,and once the-OH is protonated the molecule may take place in a variety of substitution and/or elimination reactions. The nature of R determines whether the reactions proceed via SNI or SN2 mechanisms (If R is primary alkylSN2 If R is bulky tertiary alkyl->SN1). ChlI Reacns of Alcohols (landscape) Page 9Ch11 Reacns of Alcohols (landscape) Page 9 Alcohols and Hydrohaloic Acids Alkyl halides can also be formed by reaction of alcohols with H-X acids. R-OH + H-Br R-Br + H2O In acidic media, the alcohol is in equilibrium with its protonated form. The –OH is a poor leaving group, but –OH2 + is an excellent leaving group, and once the -OH is protonated the molecule may take place in a variety of substitution and/or elimination reactions. The nature of R determines whether the reactions proceed via SN1 or SN2 mechanisms. (If R is primary alkyl SN2 If R is bulky tertiary alkyl SN1)