正在加载图片...
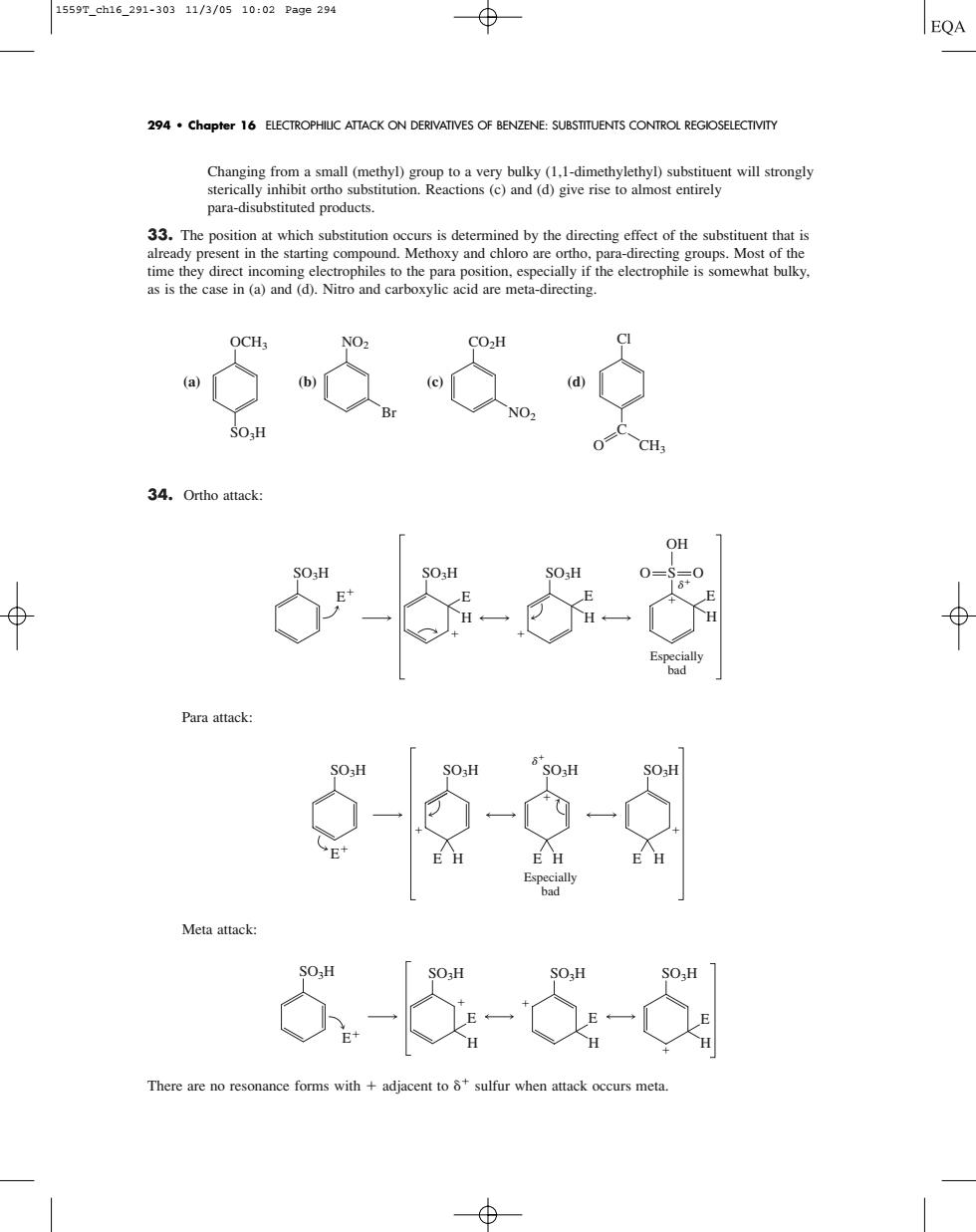
1559T_ch16_291-30311/3/0510:02Page294 ⊕ EQA 294.Chapter 16 ELECTROPHIUC ATTACK ON DERIVATIVES OF BENZENE:SUBSTITUENTS CONTROL REGIOSELECTIVITY Changing m a small (methyl g para-disubstituted products. s is determined by the directing effect of the substit t that is 式69 34.Ortho attack 6-心-5 反 6--6- There are no resonanc forms with+adjacent tosulfur when attack occurs me Changing from a small (methyl) group to a very bulky (1,1-dimethylethyl) substituent will strongly sterically inhibit ortho substitution. Reactions (c) and (d) give rise to almost entirely para-disubstituted products. 33. The position at which substitution occurs is determined by the directing effect of the substituent that is already present in the starting compound. Methoxy and chloro are ortho, para-directing groups. Most of the time they direct incoming electrophiles to the para position, especially if the electrophile is somewhat bulky, as is the case in (a) and (d). Nitro and carboxylic acid are meta-directing. (a) (b) (c) (d) 34. Ortho attack: Para attack: Meta attack: There are no resonance forms with adjacent to sulfur when attack occurs meta. SO3H E SO3H E H SO3H SO3H E E H H SO3H E SO3H SO3H E H Especially bad E H SO3H E H SO3H E SO3H E H SO3H E H S OH O O E H Especially bad Cl C O CH3 CO2H NO2 NO2 Br OCH3 SO3H 294 • Chapter 16 ELECTROPHILIC ATTACK ON DERIVATIVES OF BENZENE: SUBSTITUENTS CONTROL REGIOSELECTIVITY 1559T_ch16_291-303 11/3/05 10:02 Page 294����������������