正在加载图片...
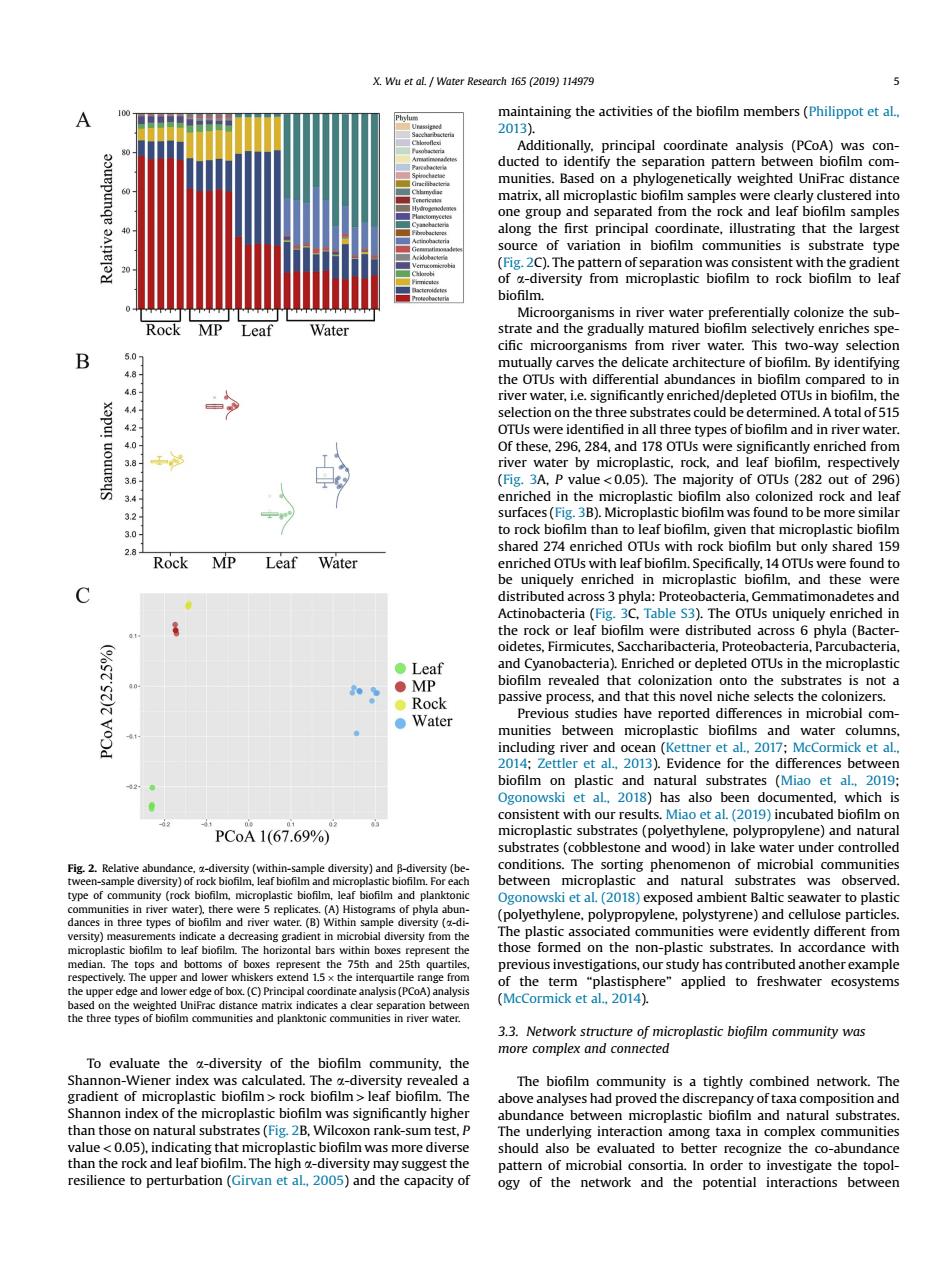
Wu et al./Water Research 165 (2019)11497 A taining the activities of the biofilm members(Phiippotetal ix.alsed on a p the ro variation bioflm communities is substrate typ -diversity from microplastic biofilm to rock biofm to lea Rock MP ganisms in river water preferentially colonize the sub Leaf Water nd the n vely en water This sele tecture By Ic ater.Le .significantly enriched/ dep eted oTUs s were i hiedinathree e types of bi ofilm and in water also rock and han Rock MP with rock Leaf hared 2 m but only sh Wate ely enri microp stic biofm an teria( uely enriched i ed across 6 p. ●Leaf onto rat at this nov ects Water micr and a)Evidence for the differe h PCoA1(67.69% our ne nd wood)I er tes ne,polypropylene,polystyrene)and cellu e particle The he formed on the pon-plastic substrates. cordance with f the term the The biofilo proved thedisc y oftaxa co than those on natural substrates (W xon rank-sum test. The under ersity may sugge ld of mic to a.In order capacity o ogy of the network and the potential interactions betweenTo evaluate the a-diversity of the biofilm community, the Shannon-Wiener index was calculated. The a-diversity revealed a gradient of microplastic biofilm > rock biofilm > leaf biofilm. The Shannon index of the microplastic biofilm was significantly higher than those on natural substrates (Fig. 2B, Wilcoxon rank-sum test, P value < 0.05), indicating that microplastic biofilm was more diverse than the rock and leaf biofilm. The high a-diversity may suggest the resilience to perturbation (Girvan et al., 2005) and the capacity of maintaining the activities of the biofilm members (Philippot et al., 2013). Additionally, principal coordinate analysis (PCoA) was conducted to identify the separation pattern between biofilm communities. Based on a phylogenetically weighted UniFrac distance matrix, all microplastic biofilm samples were clearly clustered into one group and separated from the rock and leaf biofilm samples along the first principal coordinate, illustrating that the largest source of variation in biofilm communities is substrate type (Fig. 2C). The pattern of separation was consistent with the gradient of a-diversity from microplastic biofilm to rock biofilm to leaf biofilm. Microorganisms in river water preferentially colonize the substrate and the gradually matured biofilm selectively enriches specific microorganisms from river water. This two-way selection mutually carves the delicate architecture of biofilm. By identifying the OTUs with differential abundances in biofilm compared to in river water, i.e. significantly enriched/depleted OTUs in biofilm, the selection on the three substrates could be determined. A total of 515 OTUs were identified in all three types of biofilm and in river water. Of these, 296, 284, and 178 OTUs were significantly enriched from river water by microplastic, rock, and leaf biofilm, respectively (Fig. 3A, P value < 0.05). The majority of OTUs (282 out of 296) enriched in the microplastic biofilm also colonized rock and leaf surfaces (Fig. 3B). Microplastic biofilm was found to be more similar to rock biofilm than to leaf biofilm, given that microplastic biofilm shared 274 enriched OTUs with rock biofilm but only shared 159 enriched OTUs with leaf biofilm. Specifically, 14 OTUs were found to be uniquely enriched in microplastic biofilm, and these were distributed across 3 phyla: Proteobacteria, Gemmatimonadetes and Actinobacteria (Fig. 3C, Table S3). The OTUs uniquely enriched in the rock or leaf biofilm were distributed across 6 phyla (Bacteroidetes, Firmicutes, Saccharibacteria, Proteobacteria, Parcubacteria, and Cyanobacteria). Enriched or depleted OTUs in the microplastic biofilm revealed that colonization onto the substrates is not a passive process, and that this novel niche selects the colonizers. Previous studies have reported differences in microbial communities between microplastic biofilms and water columns, including river and ocean (Kettner et al., 2017; McCormick et al., 2014; Zettler et al., 2013). Evidence for the differences between biofilm on plastic and natural substrates (Miao et al., 2019; Ogonowski et al., 2018) has also been documented, which is consistent with our results. Miao et al. (2019) incubated biofilm on microplastic substrates (polyethylene, polypropylene) and natural substrates (cobblestone and wood) in lake water under controlled conditions. The sorting phenomenon of microbial communities between microplastic and natural substrates was observed. Ogonowski et al. (2018) exposed ambient Baltic seawater to plastic (polyethylene, polypropylene, polystyrene) and cellulose particles. The plastic associated communities were evidently different from those formed on the non-plastic substrates. In accordance with previous investigations, our study has contributed another example of the term “plastisphere” applied to freshwater ecosystems (McCormick et al., 2014). 3.3. Network structure of microplastic biofilm community was more complex and connected The biofilm community is a tightly combined network. The above analyses had proved the discrepancy of taxa composition and abundance between microplastic biofilm and natural substrates. The underlying interaction among taxa in complex communities should also be evaluated to better recognize the co-abundance pattern of microbial consortia. In order to investigate the topology of the network and the potential interactions between Fig. 2. Relative abundance, a-diversity (within-sample diversity) and b-diversity (between-sample diversity) of rock biofilm, leaf biofilm and microplastic biofilm. For each type of community (rock biofilm, microplastic biofilm, leaf biofilm and planktonic communities in river water), there were 5 replicates. (A) Histograms of phyla abundances in three types of biofilm and river water. (B) Within sample diversity (a-diversity) measurements indicate a decreasing gradient in microbial diversity from the microplastic biofilm to leaf biofilm. The horizontal bars within boxes represent the median. The tops and bottoms of boxes represent the 75th and 25th quartiles, respectively. The upper and lower whiskers extend 1.5 the interquartile range from the upper edge and lower edge of box. (C) Principal coordinate analysis (PCoA) analysis based on the weighted UniFrac distance matrix indicates a clear separation between the three types of biofilm communities and planktonic communities in river water. X. Wu et al. / Water Research 165 (2019) 114979 5�