正在加载图片...
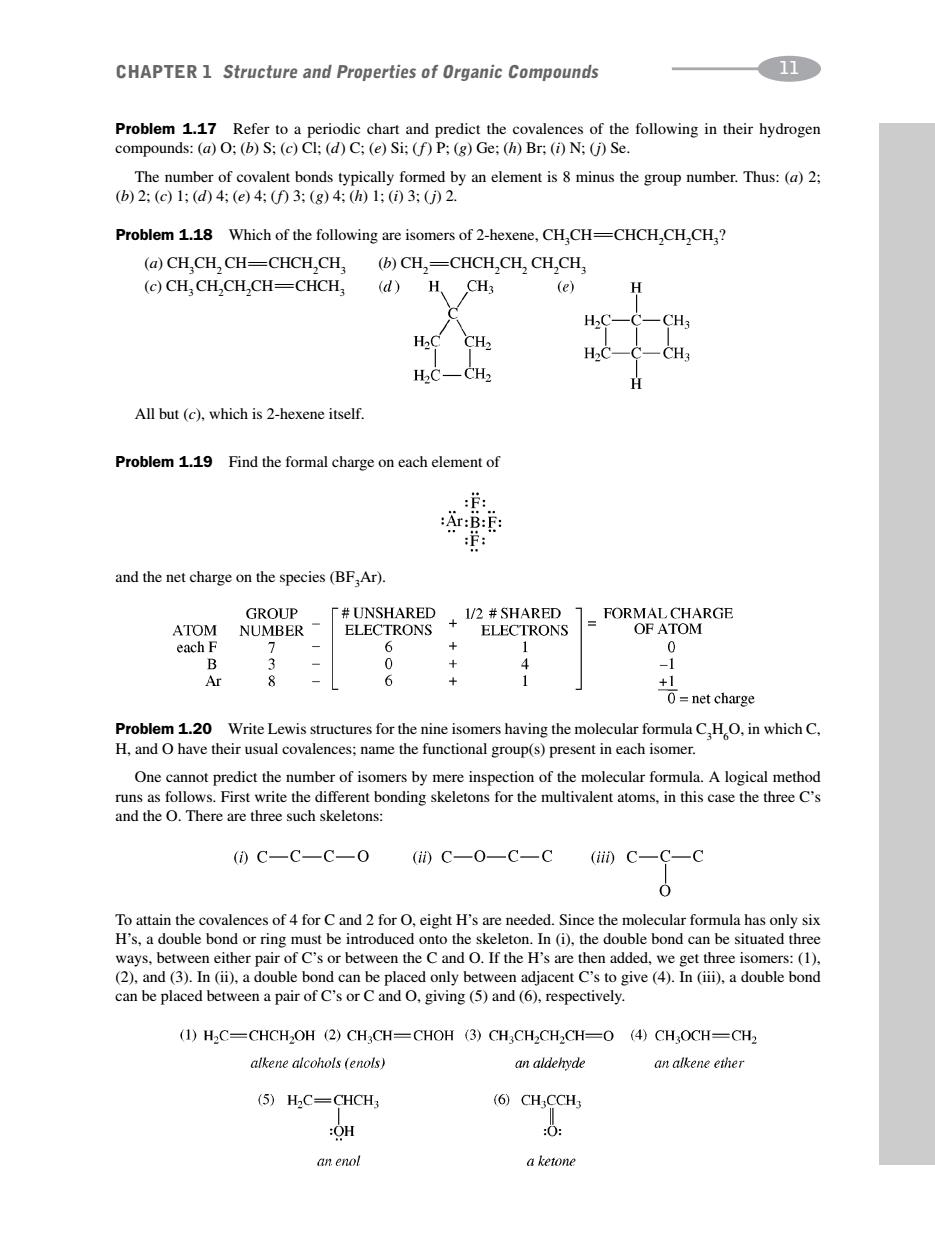
CHAPTER 1 Structure and Properties of Organic Compounds 11 Problem 1.17 Refer to a periodic chart and predict the covalences of the following in their hydrogen compounds:(a)O;(b)S;(c)Cl;(d)C;(e)Si;(f)P;(g)Ge:(h)Br:(i)N;(j)Se. The number of covalent bonds typically formed by an element is 8 minus the group number.Thus:(a)2: (b)2:(c)1;(d)4:(e)4:(f3:(g)4:(h1:()3:(j)2. Problem 1.18 Which of the following are isomers of 2-hexene,CH,CH-CHCH,CH,CH? (a)CH.CH,CH-CHCH,CH, (b)CH,=CHCH,CH,CH,CH, (c)CH,CH,CH,CH=CHCH, (d) H CH3 (e) H2C CH2 HC-CH2 All but(c),which is 2-hexene itself. Problem 1.19 Find the formal charge on each element of :f: :Ar:B:E and the net charge on the species (BF,Ar). GROUP 厂#UNSHARED ATOM NUMBER ELECTRONS +1/2 SHARED FORMAL CHARGE ELECTRONS OF ATOM each F + 1 0 Ar 8 6 1 Problem 1.20 Write Lewis structures for the nine isomers having the molecular formula C,HO,in which C, H,and O have their usual covalences;name the functional group(s)present in each isomer. One cannot predict the number of isomers by mere inspection of the molecular formula.A logical method runs as follows.First write the different bonding skeletons for the multivalent atoms,in this case the three C's and the O.There are three such skeletons: ()C一C—C一O(i)C一O—C一C(ii0C一C—C 0 To attain the covalences of 4 for C and 2 for O,eight H's are needed.Since the molecular formula has only six H's,a double bond or ring must be introduced onto the skeleton.In (i),the double bond can be situated three ways,between either pair of C's or between the C and O.If the H's are then added,we get three isomers:(1), (2).and (3).In (ii),a double bond can be placed only between adjacent C's to give(4).In (iii),a double bond can be placed between a pair of C's or Cand O.giving (5)and(6).respectively. (1)H2C=CHCH2OH(2)CH3CH=CHOH (3)CHCH2CH2CH=0 (4)CHOCH=CH2 alkene alcohols (enols) an aldehyde an alkene ether (5)H2C=CHCH3 (6)CH;CCH3 :OH :0 an enol aketoneProblem 1.17 Refer to a periodic chart and predict the covalences of the following in their hydrogen compounds: (a) O; (b) S; (c) Cl; (d) C; (e) Si; (f) P; (g) Ge; (h) Br; (i) N; (j) Se. The number of covalent bonds typically formed by an element is 8 minus the group number. Thus: (a) 2; (b) 2; (c) 1; (d) 4; (e) 4; (f) 3; (g) 4; (h) 1; (i) 3; (j) 2. Problem 1.18 Which of the following are isomers of 2-hexene, CH3CHCHCH2 CH2CH3? (a) CH3CH2CHCHCH2CH3 (b) CH2 CHCH2CH2 CH2CH3 (c) CH3CH2CH2CHCHCH3 All but (c), which is 2-hexene itself. Problem 1.19 Find the formal charge on each element of CHAPTER 1 Structure and Properties of Organic Compounds 11 and the net charge on the species (BF3 Ar). Problem 1.20 Write Lewis structures for the nine isomers having the molecular formula C3H6O, in which C, H, and O have their usual covalences; name the functional group(s) present in each isomer. One cannot predict the number of isomers by mere inspection of the molecular formula. A logical method runs as follows. First write the different bonding skeletons for the multivalent atoms, in this case the three C’s and the O. There are three such skeletons: To attain the covalences of 4 for C and 2 for O, eight H’s are needed. Since the molecular formula has only six H’s, a double bond or ring must be introduced onto the skeleton. In (i), the double bond can be situated three ways, between either pair of C’s or between the C and O. If the H’s are then added, we get three isomers: (1), (2), and (3). In (ii), a double bond can be placed only between adjacent C’s to give (4). In (iii), a double bond can be placed between a pair of C’s or C and O, giving (5) and (6), respectively.����