正在加载图片...
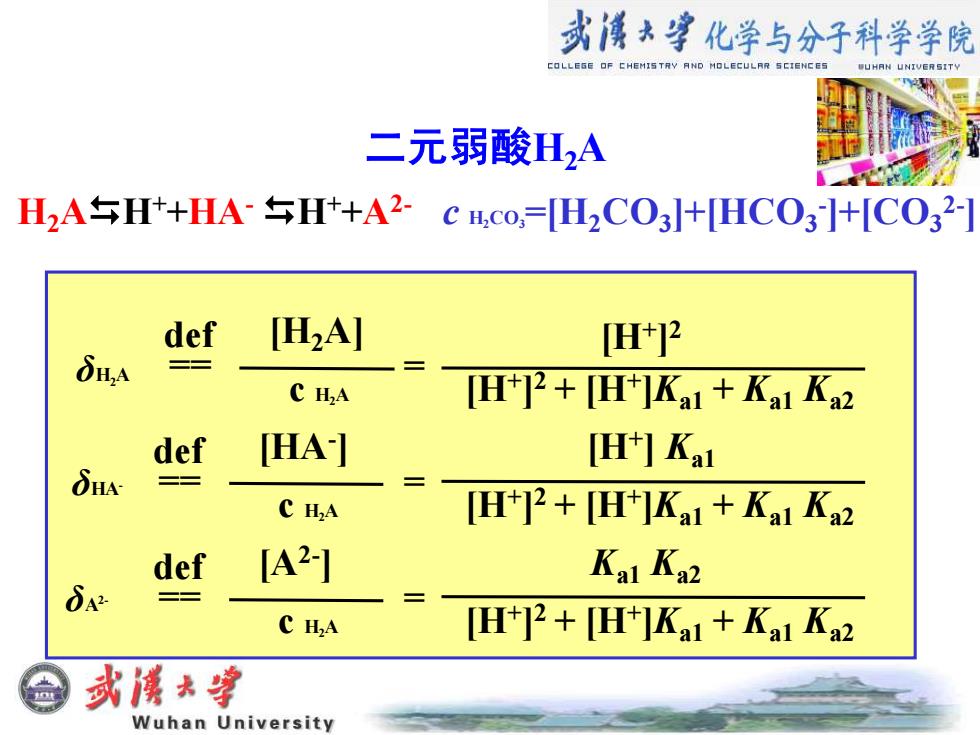
式溪*享化学与分子科学学院 COLLESE OF CHEMIS TRY AND MOLECULRR SCIENCES 二元弱酸H2A H2AH++HAH+A2-c ICo,=[H2CO3]+[HCO3]+[CO32-] def [H2A] []2 δH,A C H2A [H+2+H+]Ka1+Kal Ka2 def [HA] H中Ka1 δHN =三 C H2A [H+]2+H+]Kal+Kal Ka2 def [A2] Ka1 Ka2 C H2A [H+]2+[H+]Kal+Ka1 Ka2 或溪大穿 Wuhan University 二元弱酸H2A H2AH++HA- H++A2- c H2CO3=[H2CO3 ]+[HCO3 - ]+[CO3 2- ] [H2A] δH2A == c H2A δA2- δHAdef [HA- ] == c H2A def [A2- ] == c H2A def [H+ ] 2 [H+ ] 2 + [H+ ]Ka1 + Ka1 Ka2 = = = [H+ ] 2 + [H+ ]Ka1 + Ka1 Ka2 [H+ ] 2 + [H+ ]Ka1 + Ka1 Ka2 [H+ ] Ka1 Ka1 Ka2