正在加载图片...
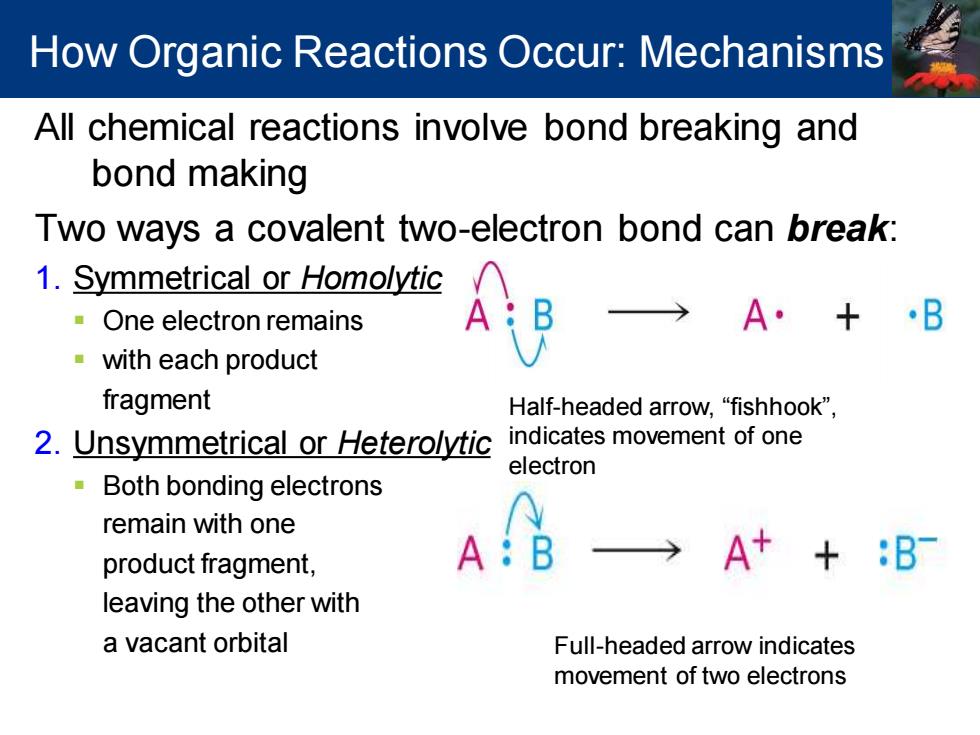
How Organic Reactions Occur:Mechanisms All chemical reactions involve bond breaking and bond making Two ways a covalent two-electron bond can break: 1.Symmetrical or Homolytic One electron remains A·+·B with each product fragment Half-headed arrow,"fishhook", 2.Unsymmetrical or Heterolytic indicates movement of one electron 。 Both bonding electrons remain with one product fragment, A A++:B leaving the other with a vacant orbital Full-headed arrow indicates movement of two electronsAll chemical reactions involve bond breaking and bond making Two ways a covalent two-electron bond can break: 1. Symmetrical or Homolytic ▪ One electron remains ▪ with each product fragment 2. Unsymmetrical or Heterolytic ▪ Both bonding electrons remain with one product fragment, leaving the other with a vacant orbital Half-headed arrow, “fishhook”, indicates movement of one electron Full-headed arrow indicates movement of two electrons How Organic Reactions Occur: Mechanisms