正在加载图片...
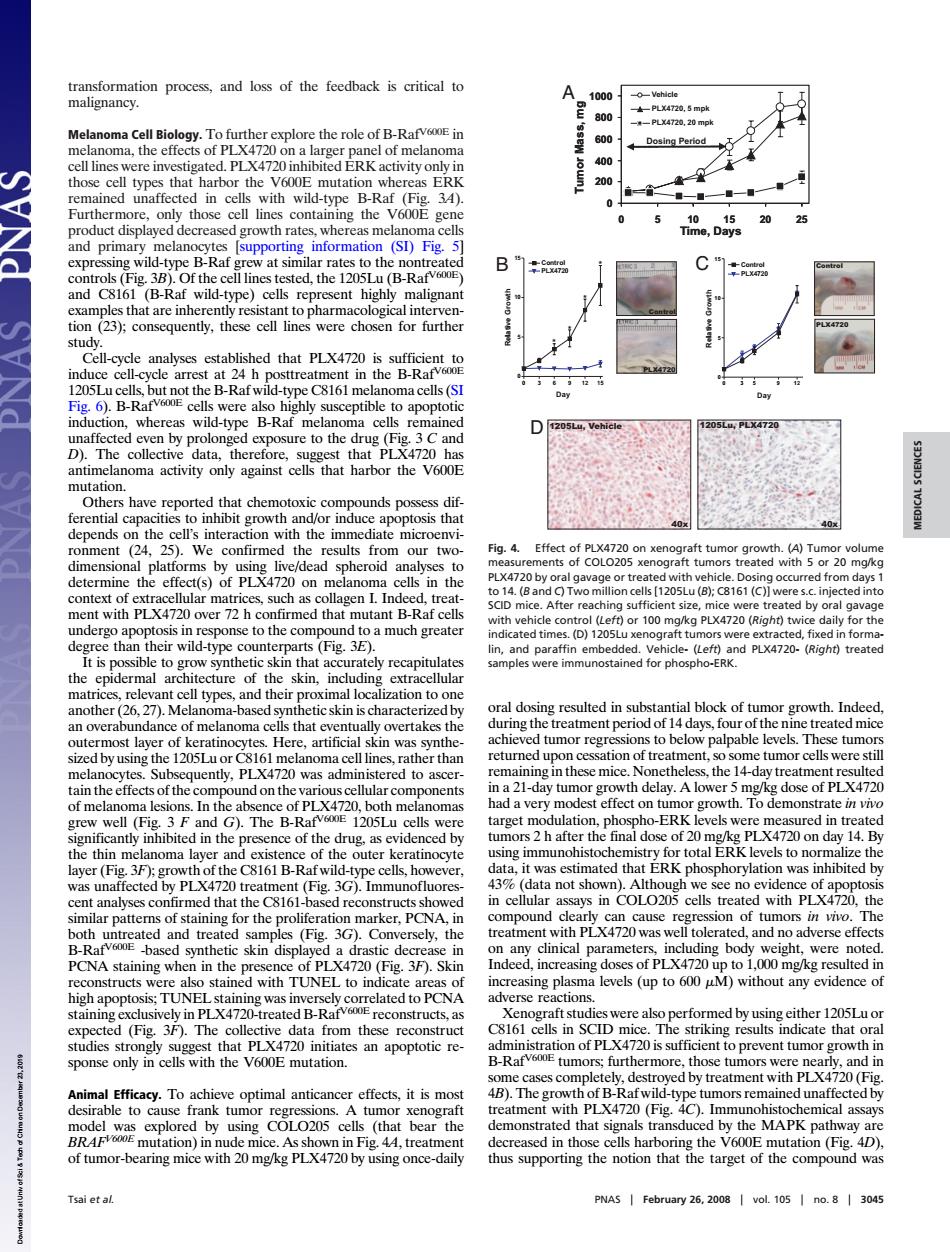
transformation proces and oss of the feedback to A fR-Ra E: the Dosing Perlod types that harbo the whereas ERK 5200 ine the 5 25 prima infor Fig 3B). B品 nes were ologicalimtecms induce cell- 4 h vere als D to the ored that che oxic co unds the the date mic sis tha ollagen I.Ind 100 LXa7201 or th in nd to a m and 1ogo ndPLX4720- treate rices rele ture and the of melanoma cells that eve ng t nt p ather than f trea nt -day tur V.A lower 5 r of Pl 1205 e latio muno Fig. not s see no c inin und au ion of tun in dis (Fig. clinical levels (up to 60 uM without an evidenc of high apoptoss T erformed byu Fig. 30 either 12051 st ation of PI X4720is t ora sponse only in cel with the V600E mutation 曲PLX4720(G B y cells (tha bear mons hat ha of tumor-bearing mice with 20 mg/kg PLX4720 by using once-daily thus supporing the notion that the target of the compound was PNAS transformation process, and loss of the feedback is critical to malignancy. Melanoma Cell Biology. To further explore the role of B-RafV600E in melanoma, the effects of PLX4720 on a larger panel of melanoma cell lines were investigated. PLX4720 inhibited ERK activity only in those cell types that harbor the V600E mutation whereas ERK remained unaffected in cells with wild-type B-Raf (Fig. 3A). Furthermore, only those cell lines containing the V600E gene product displayed decreased growth rates, whereas melanoma cells and primary melanocytes [supporting information (SI) Fig. 5] expressing wild-type B-Raf grew at similar rates to the nontreated controls (Fig. 3B). Of the cell lines tested, the 1205Lu (B-RafV600E) and C8161 (B-Raf wild-type) cells represent highly malignant examples that are inherently resistant to pharmacological intervention (23); consequently, these cell lines were chosen for further study. Cell-cycle analyses established that PLX4720 is sufficient to induce cell-cycle arrest at 24 h posttreatment in the B-RafV600E 1205Lu cells, but not the B-Raf wild-type C8161 melanoma cells (SI Fig. 6). B-RafV600E cells were also highly susceptible to apoptotic induction, whereas wild-type B-Raf melanoma cells remained unaffected even by prolonged exposure to the drug (Fig. 3 C and D). The collective data, therefore, suggest that PLX4720 has antimelanoma activity only against cells that harbor the V600E mutation. Others have reported that chemotoxic compounds possess differential capacities to inhibit growth and/or induce apoptosis that depends on the cell’s interaction with the immediate microenvironment (24, 25). We confirmed the results from our twodimensional platforms by using live/dead spheroid analyses to determine the effect(s) of PLX4720 on melanoma cells in the context of extracellular matrices, such as collagen I. Indeed, treatment with PLX4720 over 72 h confirmed that mutant B-Raf cells undergo apoptosis in response to the compound to a much greater degree than their wild-type counterparts (Fig. 3E). It is possible to grow synthetic skin that accurately recapitulates the epidermal architecture of the skin, including extracellular matrices, relevant cell types, and their proximal localization to one another (26, 27). Melanoma-based synthetic skin is characterized by an overabundance of melanoma cells that eventually overtakes the outermost layer of keratinocytes. Here, artificial skin was synthesized by using the 1205Lu or C8161 melanoma cell lines, rather than melanocytes. Subsequently, PLX4720 was administered to ascertain the effects of the compound on the various cellular components of melanoma lesions. In the absence of PLX4720, both melanomas grew well (Fig. 3 F and G). The B-RafV600E 1205Lu cells were significantly inhibited in the presence of the drug, as evidenced by the thin melanoma layer and existence of the outer keratinocyte layer (Fig. 3F); growth of the C8161 B-Raf wild-type cells, however, was unaffected by PLX4720 treatment (Fig. 3G). Immunofluorescent analyses confirmed that the C8161-based reconstructs showed similar patterns of staining for the proliferation marker, PCNA, in both untreated and treated samples (Fig. 3G). Conversely, the B-RafV600E -based synthetic skin displayed a drastic decrease in PCNA staining when in the presence of PLX4720 (Fig. 3F). Skin reconstructs were also stained with TUNEL to indicate areas of high apoptosis; TUNEL staining was inversely correlated to PCNA staining exclusively in PLX4720-treated B-RafV600E reconstructs, as expected (Fig. 3F). The collective data from these reconstruct studies strongly suggest that PLX4720 initiates an apoptotic response only in cells with the V600E mutation. Animal Efficacy. To achieve optimal anticancer effects, it is most desirable to cause frank tumor regressions. A tumor xenograft model was explored by using COLO205 cells (that bear the BRAFV600E mutation) in nude mice. As shown in Fig. 4A, treatment of tumor-bearing mice with 20 mg/kg PLX4720 by using once-daily oral dosing resulted in substantial block of tumor growth. Indeed, during the treatment period of 14 days, four of the nine treated mice achieved tumor regressions to below palpable levels. These tumors returned upon cessation of treatment, so some tumor cells were still remaining in these mice. Nonetheless, the 14-day treatment resulted in a 21-day tumor growth delay. A lower 5 mg/kg dose of PLX4720 had a very modest effect on tumor growth. To demonstrate in vivo target modulation, phospho-ERK levels were measured in treated tumors 2 h after the final dose of 20 mg/kg PLX4720 on day 14. By using immunohistochemistry for total ERK levels to normalize the data, it was estimated that ERK phosphorylation was inhibited by 43% (data not shown). Although we see no evidence of apoptosis in cellular assays in COLO205 cells treated with PLX4720, the compound clearly can cause regression of tumors in vivo. The treatment with PLX4720 was well tolerated, and no adverse effects on any clinical parameters, including body weight, were noted. Indeed, increasing doses of PLX4720 up to 1,000 mg/kg resulted in increasing plasma levels (up to 600 M) without any evidence of adverse reactions. Xenograft studies were also performed by using either 1205Lu or C8161 cells in SCID mice. The striking results indicate that oral administration of PLX4720 is sufficient to prevent tumor growth in B-RafV600E tumors; furthermore, those tumors were nearly, and in some cases completely, destroyed by treatment with PLX4720 (Fig. 4B). The growth of B-Raf wild-type tumors remained unaffected by treatment with PLX4720 (Fig. 4C). Immunohistochemical assays demonstrated that signals transduced by the MAPK pathway are decreased in those cells harboring the V600E mutation (Fig. 4D), thus supporting the notion that the target of the compound was 40x 1205Lu, Vehicle 40x D 1205Lu, PLX4720 0 3 5 9 12 0 5 10 15 Control PLX4720 Day Relative Growth 0 3 6 9 12 15 0 5 10 15 Control PLX4720 Day Re al tive Growth Control PLX4720 Control PLX4720 B C * * * * A 0 200 400 600 800 1000 0 5 10 15 20 25 Vehicle PLX4720, 5 mpk PLX4720, 20 mpk Time, Days Tumor Mass, mg Dosing Period 0 200 400 600 800 1000 0 5 10 15 20 25 Vehicle PLX4720, 5 mpk PLX4720, 20 mpk Time, Days Tumor Mass, mg Dosing Period Fig. 4. Effect of PLX4720 on xenograft tumor growth. (A) Tumor volume measurements of COLO205 xenograft tumors treated with 5 or 20 mg/kg PLX4720 by oral gavage or treated with vehicle. Dosing occurred from days 1 to 14. (B and C) Two million cells [1205Lu (B); C8161 (C)] were s.c. injected into SCID mice. After reaching sufficient size, mice were treated by oral gavage with vehicle control (Left) or 100 mg/kg PLX4720 (Right) twice daily for the indicated times. (D) 1205Lu xenograft tumors were extracted, fixed in formalin, and paraffin embedded. Vehicle- (Left) and PLX4720- (Right) treated samples were immunostained for phospho-ERK. Tsai et al. PNAS February 26, 2008 vol. 105 no. 8 3045 MEDICAL SCIENCES Downloaded at Univ of Sci & Tech of China on December 23, 2019