正在加载图片...
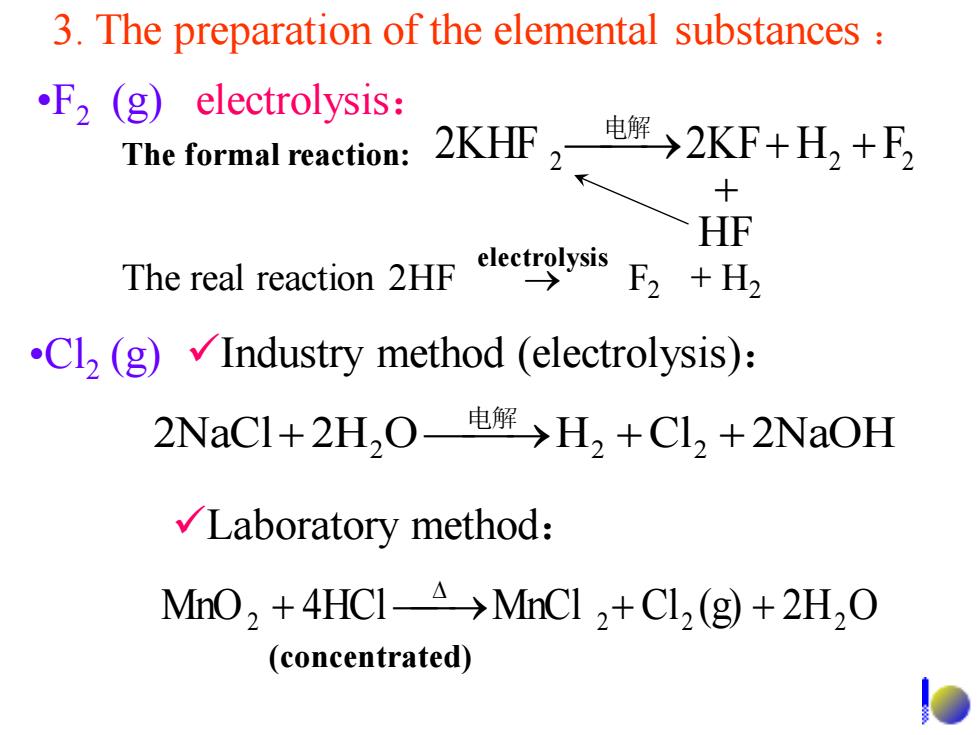
3.The preparation of the elemental substances F2 (g)electrolysis: The formal reaction: 2KHF2电→2KF+H,+E 十 HF The real reaction 2HFs ysis F2 +H2 Cl2(g)Industry method (electrolysis): 2NaCl+2H,0电解)>H2+CI2+2NaOH √Laboratory method: MnO,+4HCI-A >MnCl 2+Cl2 (g)+2H2O (concentrated) •Cl2 (g) 2NaCl 2H O H2 Cl2 2NaOH 电解 + 2 ⎯⎯→ + + ✓Laboratory method: MnO2 + 4HCl ⎯→MnCl 2 + Cl2 (g) + 2H2 O ✓Industry method (electrolysis): 3. The preparation of the elemental substances : (concentrated) •F2 (g) electrolysis: 2 2 电解 2KHF 2 ⎯⎯→2KF+ H + F + HF The real reaction 2HF → F2 + H2 The formal reaction: electrolysis