正在加载图片...
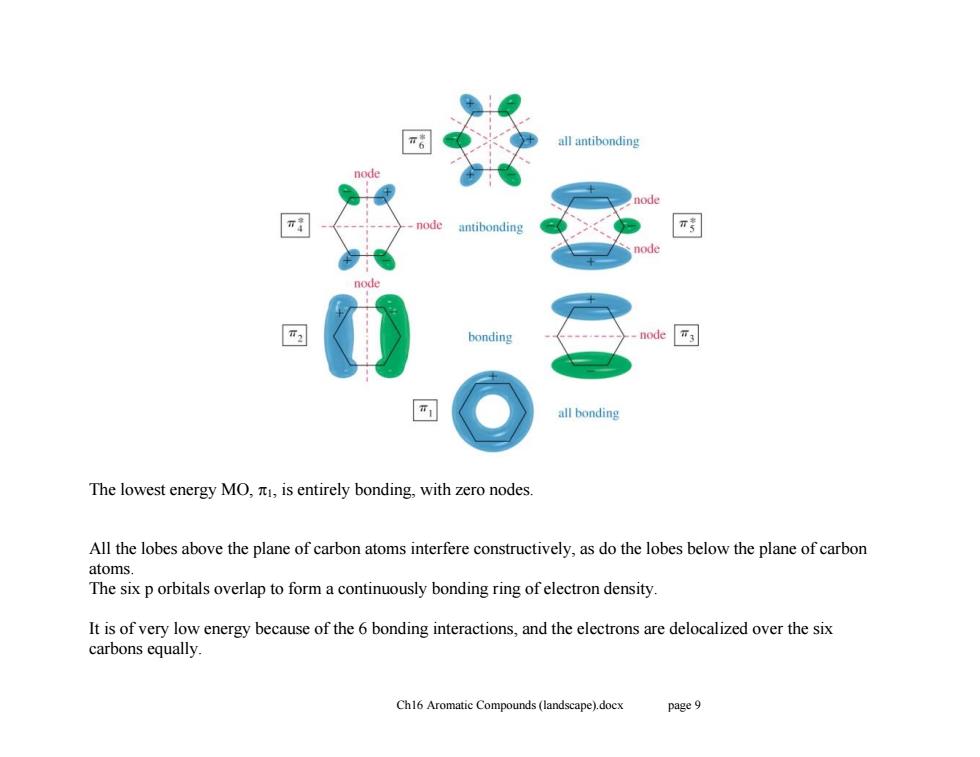
all antibonding antibonding π ode bonding node T3 all bonding The lowest energy MO,is entirely bonding,with zero nodes. All the lobes above the plane of carbon atoms interfere constructively,as do the lobes below the plane of carbon atoms. The six p orbitals overlap to form a continuously bonding ring of electron density. It is of very low energy because of the 6 bonding interactions,and the electrons are delocalized over the six carbons equally. Ch16 Aromatic Compounds(landscape).docx page 9 Ch16 Aromatic Compounds (landscape).docx page 9 The lowest energy MO, 1, is entirely bonding, with zero nodes. All the lobes above the plane of carbon atoms interfere constructively, as do the lobes below the plane of carbon atoms. The six p orbitals overlap to form a continuously bonding ring of electron density. It is of very low energy because of the 6 bonding interactions, and the electrons are delocalized over the six carbons equally