正在加载图片...
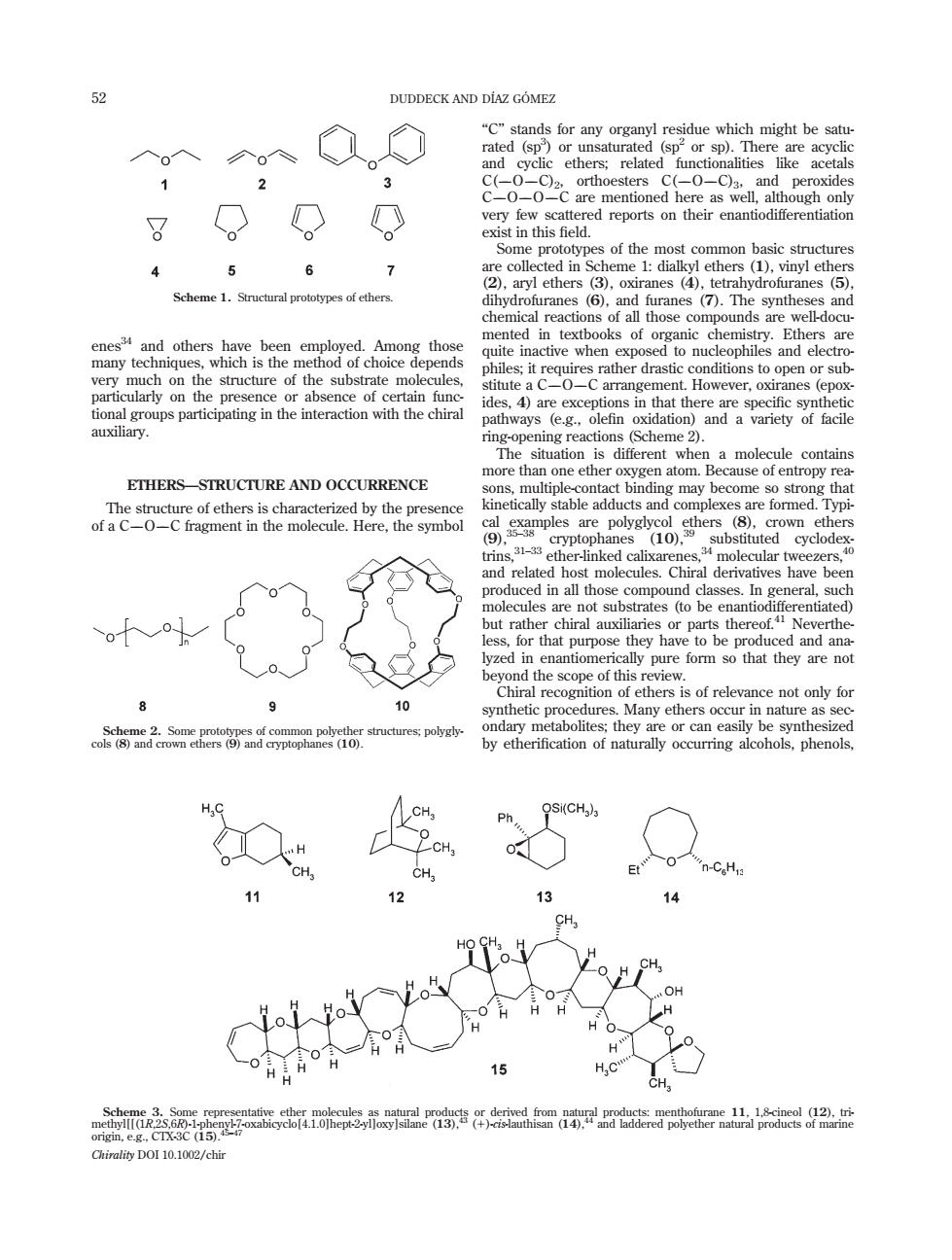
DUDDECK AND DIAZ GOMEZ 2 3 。 O very few exist int s of the most common basic structures g 5 6 7 d in S synthe enes and others have been employed.Among thos ted in textbooks of organic chemi of choice depends whe o nu nt.However,oxi ranes (epox mta微 molecule contain more use of entropy ETHERS-STRUCTURE AND OCCURRENCE than one ether oxygen atom. T kinetically stable adducts and complexes are formed.Typ ther-inked molecular tweezers d in all thos neral. purpos they hav to be prha icedandrand are e of this view M of rele dary r e or can e 12 15 盖益盒部应点威品 Chirality DOI 10.1002/chir enes34 and others have been employed. Among those many techniques, which is the method of choice depends very much on the structure of the substrate molecules, particularly on the presence or absence of certain functional groups participating in the interaction with the chiral auxiliary. ETHERS—STRUCTURE AND OCCURRENCE The structure of ethers is characterized by the presence of a COC fragment in the molecule. Here, the symbol ‘‘C’’ stands for any organyl residue which might be saturated (sp3 ) or unsaturated (sp2 or sp). There are acyclic and cyclic ethers; related functionalities like acetals C(OC)2, orthoesters C(OC)3, and peroxides COOC are mentioned here as well, although only very few scattered reports on their enantiodifferentiation exist in this field. Some prototypes of the most common basic structures are collected in Scheme 1: dialkyl ethers (1), vinyl ethers (2), aryl ethers (3), oxiranes (4), tetrahydrofuranes (5), dihydrofuranes (6), and furanes (7). The syntheses and chemical reactions of all those compounds are well-documented in textbooks of organic chemistry. Ethers are quite inactive when exposed to nucleophiles and electrophiles; it requires rather drastic conditions to open or substitute a COC arrangement. However, oxiranes (epoxides, 4) are exceptions in that there are specific synthetic pathways (e.g., olefin oxidation) and a variety of facile ring-opening reactions (Scheme 2). The situation is different when a molecule contains more than one ether oxygen atom. Because of entropy reasons, multiple-contact binding may become so strong that kinetically stable adducts and complexes are formed. Typical examples are polyglycol ethers (8), crown ethers (9),35–38 cryptophanes (10),39 substituted cyclodextrins,31–33 ether-linked calixarenes,34 molecular tweezers,40 and related host molecules. Chiral derivatives have been produced in all those compound classes. In general, such molecules are not substrates (to be enantiodifferentiated) but rather chiral auxiliaries or parts thereof.41 Nevertheless, for that purpose they have to be produced and analyzed in enantiomerically pure form so that they are not beyond the scope of this review. Chiral recognition of ethers is of relevance not only for synthetic procedures. Many ethers occur in nature as secondary metabolites; they are or can easily be synthesized by etherification of naturally occurring alcohols, phenols, Scheme 1. Structural prototypes of ethers. Scheme 2. Some prototypes of common polyether structures; polyglycols (8) and crown ethers (9) and cryptophanes (10). Scheme 3. Some representative ether molecules as natural products or derived from natural products: menthofurane 11, 1,8-cineol (12), trimethyl[[(1R,2S,6R)-1-phenyl-7-oxabicyclo[4.1.0]hept-2-yl]oxy]silane (13),43 (1)-cis-lauthisan (14),44 and laddered polyether natural products of marine origin, e.g., CTX-3C (15).45–47 52 DUDDECK AND DI´AZ GO´ MEZ Chirality DOI 10.1002/chir