正在加载图片...
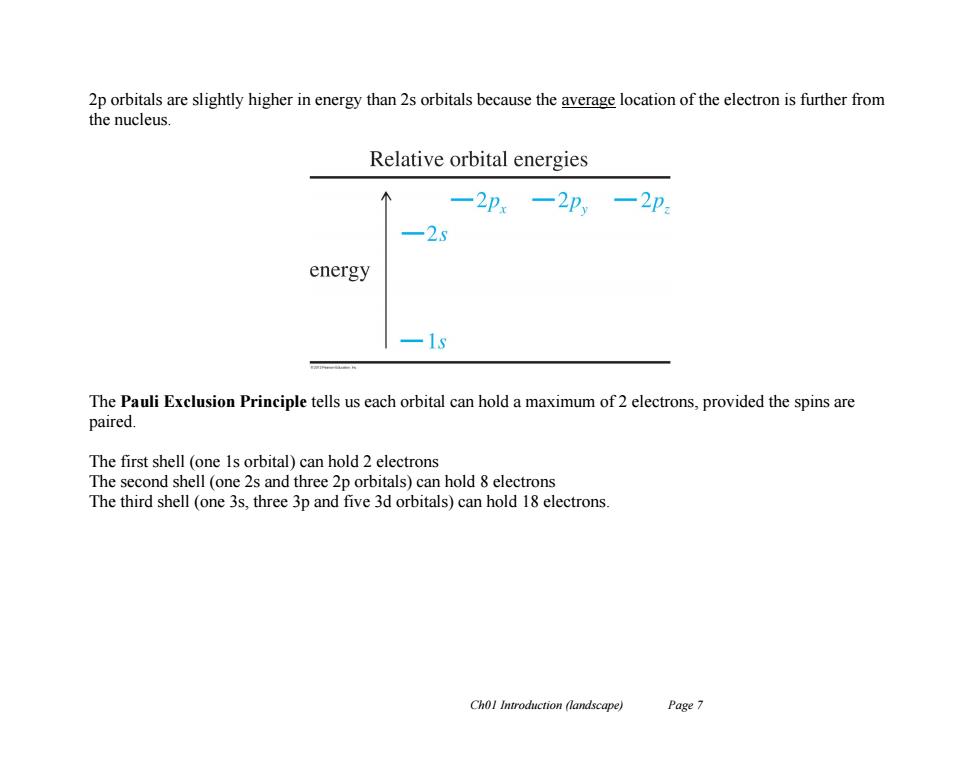
2p orbitals are slightly higher in energy than 2s orbitals because the average location of the electron is further from the nucleus. Relative orbital energies -2Px -2p,-2p -25 energy 一1 The Pauli Exclusion Principle tells us each orbital can hold a maximum of 2 electrons,provided the spins are paired. The first shell (one 1s orbital)can hold 2 electrons The second shell (one 2s and three 2p orbitals)can hold 8 electrons The third shell (one 3s.three 3p and five 3d orbitals)can hold 18 electrons. Chol Introduction (landscape) Page 7 Ch01 Introduction (landscape) Page 7 2p orbitals are slightly higher in energy than 2s orbitals because the average location of the electron is further from the nucleus. The Pauli Exclusion Principle tells us each orbital can hold a maximum of 2 electrons, provided the spins are paired. The first shell (one 1s orbital) can hold 2 electrons The second shell (one 2s and three 2p orbitals) can hold 8 electrons The third shell (one 3s, three 3p and five 3d orbitals) can hold 18 electrons