正在加载图片...
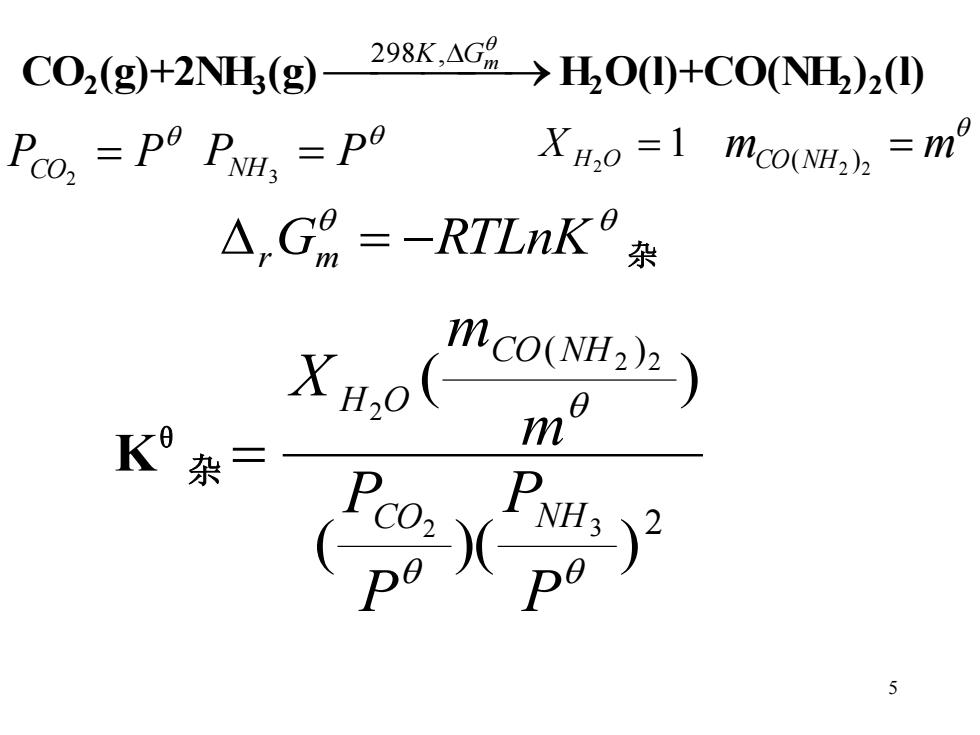
C02(g)+2NH(g) 298K.AG>HO(I)+CO(NH2)2() Peos =Po PN po XH2o =1 mCo(NH)h =m △,G9=-RTLnK杂 mco(NH22) K XH,o(n2 55 CO2 (g)+2NH3 (g)⎯⎯⎯ ⎯→ 298K, Gm H2 O(l)+CO(NH2 )2 (l) PCO = P 2 PNH = P 3 1 2 X H O = mCO NH = m 2 2 ( ) r Gm = −RTLnK 杂 Kθ 杂 2 ( ) ( )( ) ( ) 2 3 2 2 2 P P P P m m X CO NH CO NH H O =