正在加载图片...
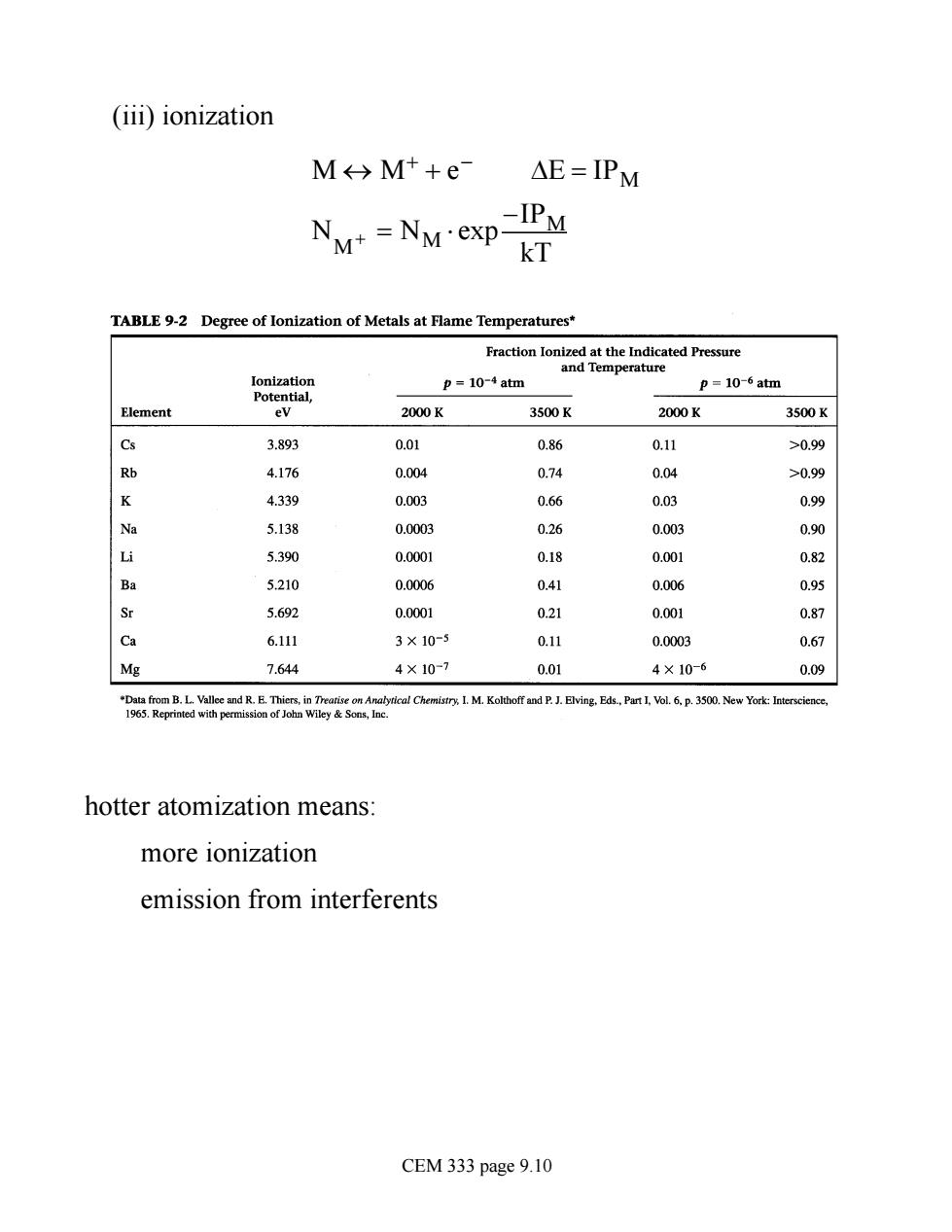
(iii))ionization M→Mt+e △E=IPM NM=NM-exp-IPM kT TABLE 92 Degree of Ionization of Metals at Flame Temperatures p=10-4atm p=10-6atm Element ev 2000K 3500K 2000K 3500K 3.893 0.01 0.86 0.11 >0.99 4.176 0.004 0.74 0.04 >0.99 K 4.339 0.003 0.66 0.03 0.99 5.138 0.0003 0.26 0.003 0.90 5.390 0.0001 0.18 .00 0.82 5.210 0.0006 0.41 0.006 0.95 Sr 5.692 0.0001 021 0.001 0.87 Ca 6.111 3×10-5 0.11 0.0003 0.67 Mg 7.644 4×10-7 0.01 4×10-6 0.09 hotter atomization means: more ionization emission from interferents CEM 333 page 9.10(iii) ionization M « M+ + e - DE = IPM NM+ = NM × exp -IPM kT hotter atomization means: more ionization emission from interferents CEM 333 page 9.10