正在加载图片...
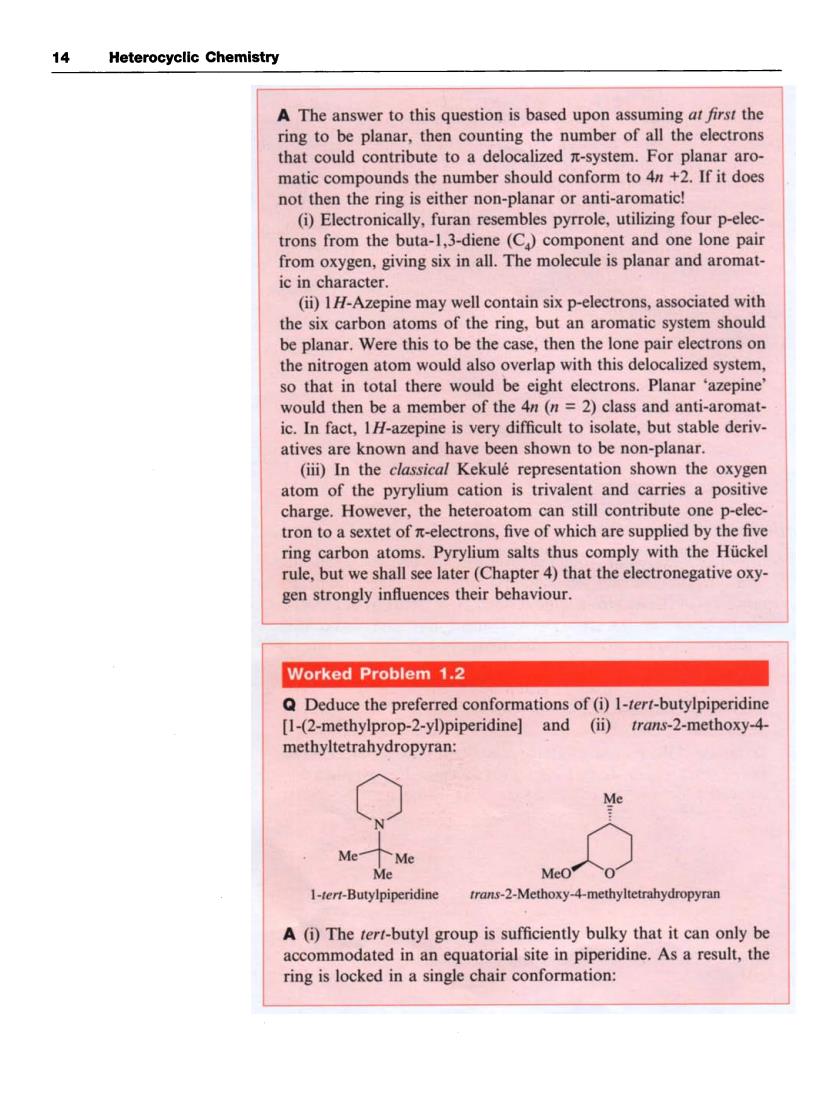
14 Heterocyclic Chemistry A The answer to this question is based upon assuming at first the ring to be planar. then cou the number of all the electrons soucontribute to deloca For planar aro matic compounds the number should conform to 4n+2.If it does not then the ring is either non-planar or anti-aromatic! (i)Electronically,furan resembles pyrrole,utilizing four p-elec trons from the buta-1,3-diene(C)component and one lone pair from oxygen,giving six in all.The molecule is planar and aromat- ic in character. (ii)1H-Azepine may well contain six p-electrons,associated with the six carbon atoms of the ring.but an aromatic system should be planar.Were this to be the case,then the lone pair electrons on the nitrogen atom would also overlap with this delocalized system so that in total there would be eight electrons.Planar 'azepine would then be a member of the 4n (n=2)class and anti-a aromat ic.In fact,IH-azepine is very difficult to isolate,but stable deriv. atives are known and have been shown to be non-planar. (iii)In the classical Kekule representation shown the oxygen atom of the pyrylium cation is trivalent and carries a posi charge.However,the heteroatom can still contribute one p-elec tron to a sextet of n-electrons,five of which are supplied by the five ring carbon atoms.Pyrylium salts thus comply with the Huckel ru but we shall see later (Chapter 4)that the gen strongly influences their behaviour. Worked Problem 1.2 Q Deduce the preferred conformations of(i)1-tert-butylpiperidine [1-(2-methylprop-2-yl)piperidine]and (ii)trans-2-methoxy-4 methyltetrahydropyran: 1-tert-Butylpiperidine trans-2-Methoxy-4-methyltetrahydropyran A (i)The tert-butyl group is sufficiently bulky that it can only be acco modated in an equatorial site in piperidine.As a result,the ring is locked in a single chair conformation: 14 Heterocyclic Chemistry