正在加载图片...
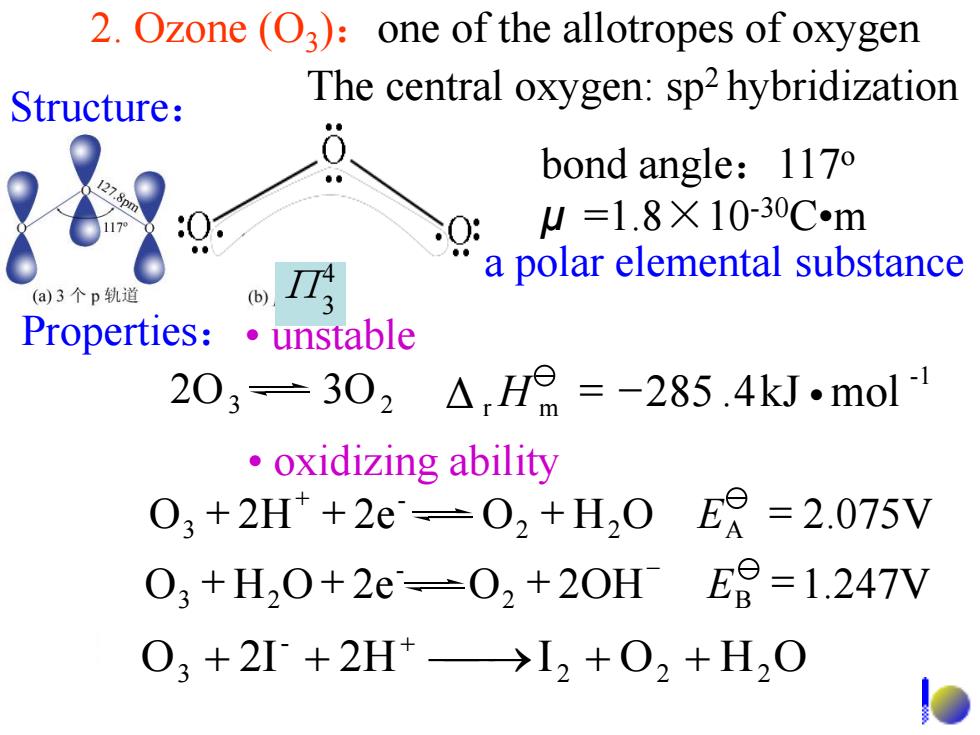
2.Ozone (O3):one of the allotropes of oxygen Structure: The central oxygen:sp2 hybridization bond angle:1170 SO: W=1.8×10-30Cm a polar elemental substance (a)3个p轨道 Properties:unstable 203=302△H=-285.4kJ.mol ·oxidizing ability 03+2H+2e-02+H,0ER=2.075V 03+H,0+2e-02+20HE9=-1.247V 03+2I+2H+→I2+02+H2O 2. Ozone (O3 ):one of the allotropes of oxygen O 2I 2H I 2 O2 H2 O - 3 + + + + + Structure: The central oxygen: sp2 hybridization bond angle:117o μ =1.8×10-30C•m Properties:• unstable • oxidizing ability a polar elemental substance 2O3 3O2 -1 Δ r H m = -285 .4kJ •mol O 2H 2e O2 H2O A 2.075V - 3 + + + = + E O H O 2e O2 2OH B 1.247V - 3 2 + + + = - E 4 Π3