正在加载图片...
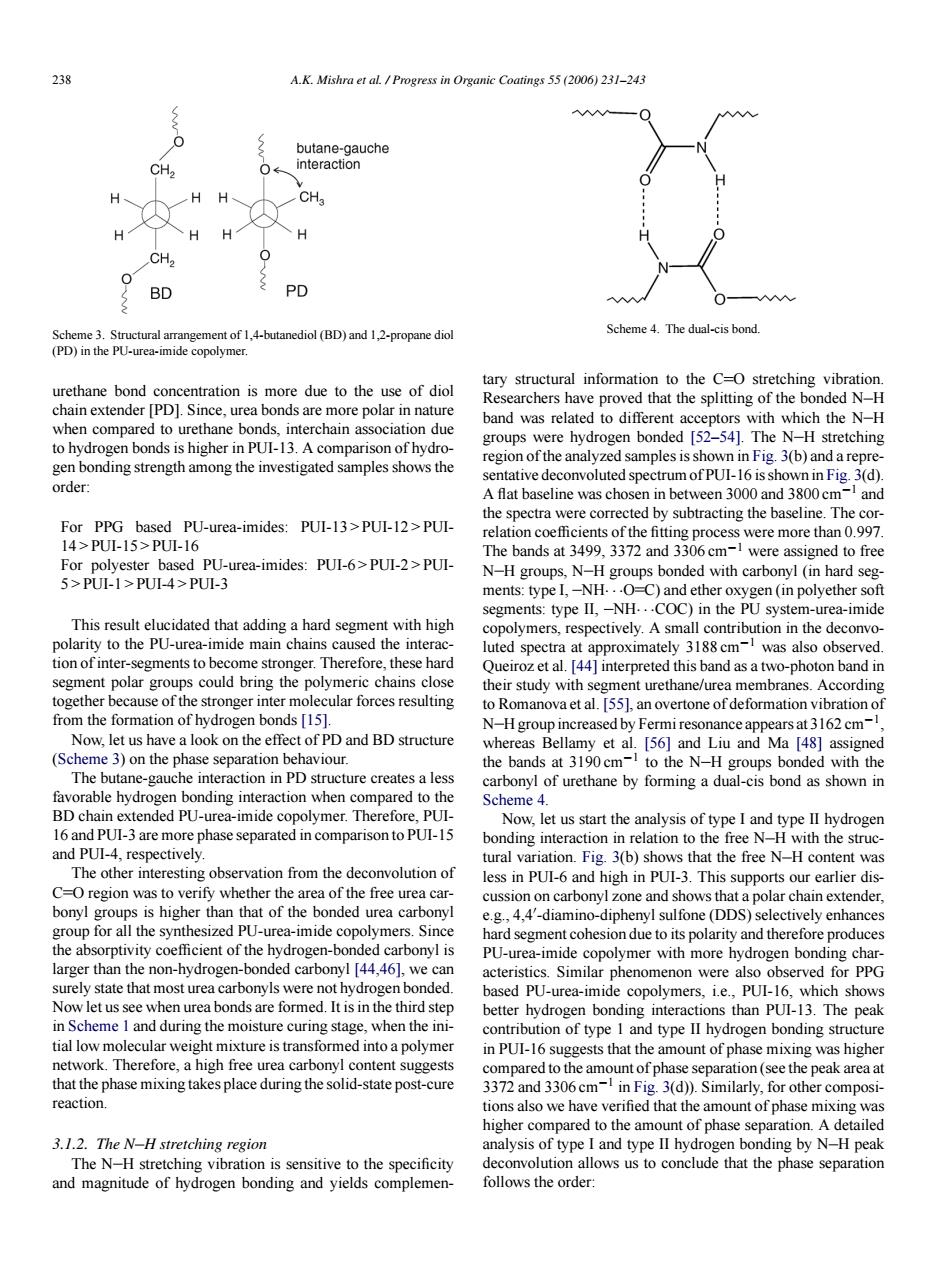
238 A.K.Mishra et al.Progress in Organic Coatings 55 (2006)231-243 H HH CH H CH BD PD Scheme4.The dual-cis bond. urethane bond concentration is more due to the use of diol tary structural information to the C-O stretching vibration. chain extender [PD].Since,urea bonds are more polar in nature earchers have proved that the splitting of the bonded N-H band was related the ne b gen bonding strength among the investigated samples shows the region of the analyzed samples is shown in Fig.3(b)and a repre order: sentative deconvoluted spectrumof PUI-16 is shown in Fig.3(d). A flat baseline was cho and 0 T FPU-wea-imides:PUI-13PU-2PUl ere more than 0.997 For sed PU-urea-imides:PUI-6>PUI-2>PUI- The bands at 499,3372 and 3306cm-1 were assigned to free 5>PULI>PUL4-PUL3 ments:type -NH This result elucidated that adding a hard segment with high copolymers respectively.A small contribution in the deconv mide main cha luted spectra at approximately 3188 cm was also observed segment polar groups could bring the polymeric chains close 4]interpreted th Ias a two-photon ba together because of the stronger inter mol cular forces resulting to Romanova etal.551 an overtone of de formation vibration o s15 (Scheme 3)on the phase s and BD structure The butane-gauche interaction in PD structure creates a less e by fo dual-is hond as shown in when compared to the Scheme 4. ted in on to PUI-Is and PUI-.respectivelv on to itn the stru The other interesting obse rvation from th deconvolution of less in PUI-6 and high in PUI-3.This sup orts our earlier dis verify whet area of山 free urea car cussion on carbonyl zone and shows th at a polar chain extender for the synthesized PU-urea-imide .4'-diamino-diphenyl sulfone (DDS) PU-urea-imide olymer with more hydr larger than the non-hydrogen-bonded carbonyl [44,46],we acteristics.Similar phenomenon were also observed for PPG Now letus se when based PU-urea-imic the third ste copolymer nich show: 11 in Scheme 1 and during the moisture curing stage when the ini he peak tial low molecular weight mixture is transformed into a polyme in PUI-16 suggests that the amount of phase mixing was higher compared to the amount of phas e separation (see the peak area at and 3306 cm compos reaction 3(d)).Similarly,for other higher compared to the amount of phase 3.1.2.The N-H stretching region analysis of type I and type lI hydrogen bonding by N-H peak s the ord to conclude that the 238 A.K. Mishra et al. / Progress in Organic Coatings 55 (2006) 231–243 Scheme 3. Structural arrangement of 1,4-butanediol (BD) and 1,2-propane diol (PD) in the PU-urea-imide copolymer. urethane bond concentration is more due to the use of diol chain extender [PD]. Since, urea bonds are more polar in nature when compared to urethane bonds, interchain association due to hydrogen bonds is higher in PUI-13. A comparison of hydrogen bonding strength among the investigated samples shows the order: For PPG based PU-urea-imides: PUI-13 > PUI-12 > PUI- 14 > PUI-15 > PUI-16 For polyester based PU-urea-imides: PUI-6 > PUI-2 > PUI- 5 > PUI-1 > PUI-4 > PUI-3 This result elucidated that adding a hard segment with high polarity to the PU-urea-imide main chains caused the interaction of inter-segments to become stronger. Therefore, these hard segment polar groups could bring the polymeric chains close together because of the stronger inter molecular forces resulting from the formation of hydrogen bonds [15]. Now, let us have a look on the effect of PD and BD structure (Scheme 3) on the phase separation behaviour. The butane-gauche interaction in PD structure creates a less favorable hydrogen bonding interaction when compared to the BD chain extended PU-urea-imide copolymer. Therefore, PUI- 16 and PUI-3 are more phase separated in comparison to PUI-15 and PUI-4, respectively. The other interesting observation from the deconvolution of C O region was to verify whether the area of the free urea carbonyl groups is higher than that of the bonded urea carbonyl group for all the synthesized PU-urea-imide copolymers. Since the absorptivity coefficient of the hydrogen-bonded carbonyl is larger than the non-hydrogen-bonded carbonyl [44,46], we can surely state that most urea carbonyls were not hydrogen bonded. Now let us see when urea bonds are formed. It is in the third step in Scheme 1 and during the moisture curing stage, when the initial low molecular weight mixture is transformed into a polymer network. Therefore, a high free urea carbonyl content suggests that the phase mixing takes place during the solid-state post-cure reaction. 3.1.2. The N H stretching region The N H stretching vibration is sensitive to the specificity and magnitude of hydrogen bonding and yields complemenScheme 4. The dual-cis bond. tary structural information to the C O stretching vibration. Researchers have proved that the splitting of the bonded N H band was related to different acceptors with which the N H groups were hydrogen bonded [52–54]. The N H stretching region of the analyzed samples is shown in Fig. 3(b) and a representative deconvoluted spectrum of PUI-16 is shown in Fig. 3(d). A flat baseline was chosen in between 3000 and 3800 cm−1 and the spectra were corrected by subtracting the baseline. The correlation coefficients of the fitting process were more than 0.997. The bands at 3499, 3372 and 3306 cm−1 were assigned to free N H groups, N H groups bonded with carbonyl (in hard segments: type I, NH···O C) and ether oxygen (in polyether soft segments: type II, NH···COC) in the PU system-urea-imide copolymers, respectively. A small contribution in the deconvoluted spectra at approximately 3188 cm−1 was also observed. Queiroz et al. [44] interpreted this band as a two-photon band in their study with segment urethane/urea membranes. According to Romanova et al. [55], an overtone of deformation vibration of N H group increased by Fermi resonance appears at 3162 cm−1, whereas Bellamy et al. [56] and Liu and Ma [48] assigned the bands at 3190 cm−1 to the N H groups bonded with the carbonyl of urethane by forming a dual-cis bond as shown in Scheme 4. Now, let us start the analysis of type I and type II hydrogen bonding interaction in relation to the free N H with the structural variation. Fig. 3(b) shows that the free N H content was less in PUI-6 and high in PUI-3. This supports our earlier discussion on carbonyl zone and shows that a polar chain extender, e.g., 4,4 -diamino-diphenyl sulfone (DDS) selectively enhances hard segment cohesion due to its polarity and therefore produces PU-urea-imide copolymer with more hydrogen bonding characteristics. Similar phenomenon were also observed for PPG based PU-urea-imide copolymers, i.e., PUI-16, which shows better hydrogen bonding interactions than PUI-13. The peak contribution of type 1 and type II hydrogen bonding structure in PUI-16 suggests that the amount of phase mixing was higher compared to the amount of phase separation (see the peak area at 3372 and 3306 cm−1 in Fig. 3(d)). Similarly, for other compositions also we have verified that the amount of phase mixing was higher compared to the amount of phase separation. A detailed analysis of type I and type II hydrogen bonding by N H peak deconvolution allows us to conclude that the phase separation follows the order:�