正在加载图片...
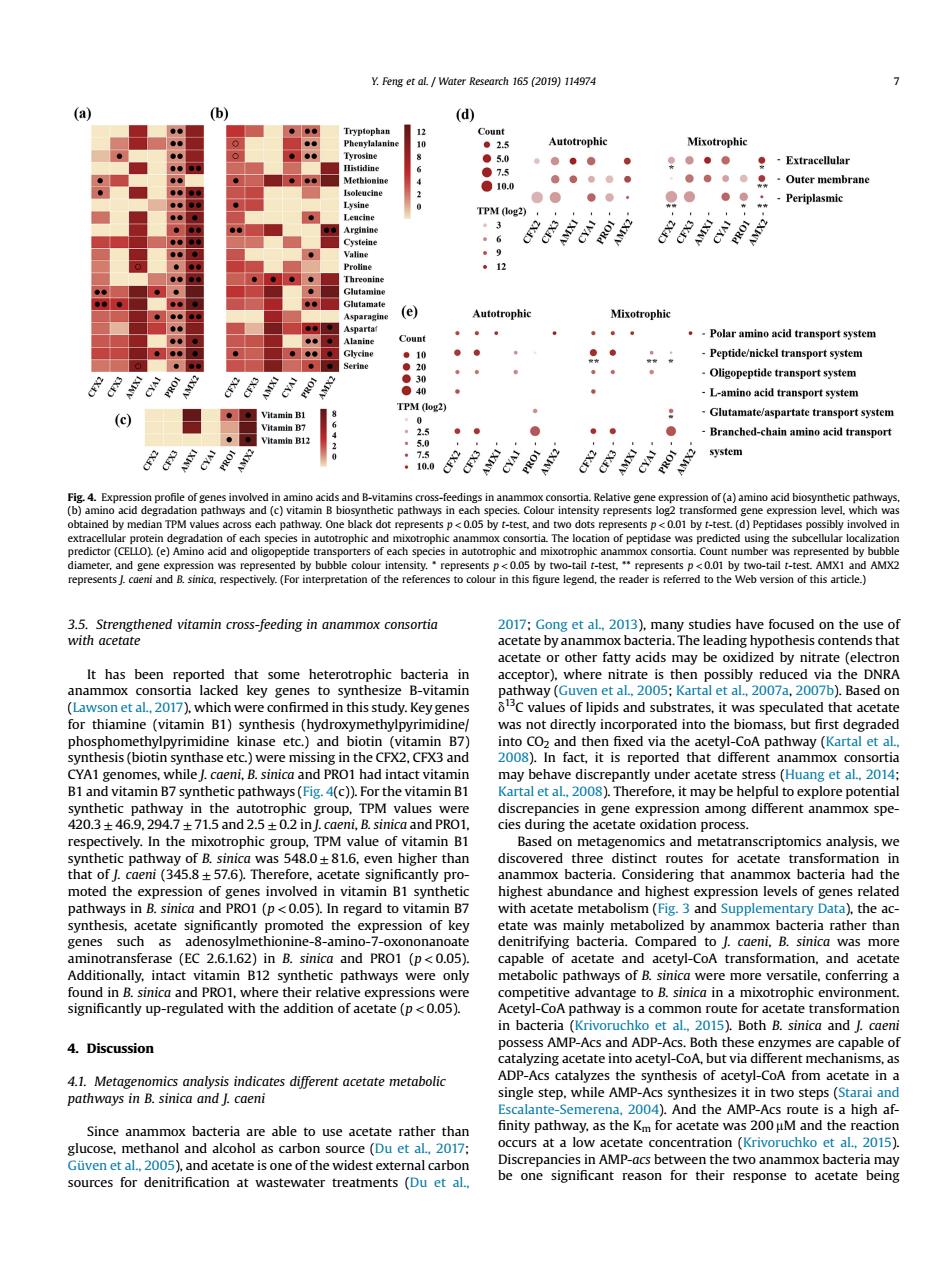
¥Feng et al./wat d Autotrophic Mixotrophic 0。。. (e) Autotrophic .-Pelar amino acid transport system el transport syste AA S Glutamate aspartate transpor c) Branched-chain amino acid transport 。 system d B-vi in cies Colou epgno acdesewdw f pep ase was sing the s LO)(e)A 0051 y-tlMXI and AM It has bee t al Kartal et al 2007 ,2007b.B for thiamine (vitamin B1) thesis orated into th s but first des in the CFX2. orti nath had intact may behave Based o a .comp of a acetate andac etyl-CoA transforma ound inB sinica and PRO1,wh ge to B.sinica in mixotrophic en n bacteria (Krivon to et al. Both B.sinica and caer 4 Discussion zing acetate into 4.1.Metagenomics analysis indicates different acetate metabolic pathways in B.sinica and I.caeni And s a high a ccurs at a concentration(Kr and acetate isone of the ridest external carbor ources for denitrification at wastewater treatments (Du et a be one significant reason for resnons to acetate heins 3.5. Strengthened vitamin cross-feeding in anammox consortia with acetate It has been reported that some heterotrophic bacteria in anammox consortia lacked key genes to synthesize B-vitamin (Lawson et al., 2017), which were confirmed in this study. Key genes for thiamine (vitamin B1) synthesis (hydroxymethylpyrimidine/ phosphomethylpyrimidine kinase etc.) and biotin (vitamin B7) synthesis (biotin synthase etc.) were missing in the CFX2, CFX3 and CYA1 genomes, while J. caeni, B. sinica and PRO1 had intact vitamin B1 and vitamin B7 synthetic pathways (Fig. 4(c)). For the vitamin B1 synthetic pathway in the autotrophic group, TPM values were 420.3 ± 46.9, 294.7 ± 71.5 and 2.5 ± 0.2 in J. caeni, B. sinica and PRO1, respectively. In the mixotrophic group, TPM value of vitamin B1 synthetic pathway of B. sinica was 548.0 ± 81.6, even higher than that of J. caeni (345.8 ± 57.6). Therefore, acetate significantly promoted the expression of genes involved in vitamin B1 synthetic pathways in B. sinica and PRO1 (p < 0.05). In regard to vitamin B7 synthesis, acetate significantly promoted the expression of key genes such as adenosylmethionine-8-amino-7-oxononanoate aminotransferase (EC 2.6.1.62) in B. sinica and PRO1 (p < 0.05). Additionally, intact vitamin B12 synthetic pathways were only found in B. sinica and PRO1, where their relative expressions were significantly up-regulated with the addition of acetate (p < 0.05). 4. Discussion 4.1. Metagenomics analysis indicates different acetate metabolic pathways in B. sinica and J. caeni Since anammox bacteria are able to use acetate rather than glucose, methanol and alcohol as carbon source (Du et al., 2017; Güven et al., 2005), and acetate is one of the widest external carbon sources for denitrification at wastewater treatments (Du et al., 2017; Gong et al., 2013), many studies have focused on the use of acetate by anammox bacteria. The leading hypothesis contends that acetate or other fatty acids may be oxidized by nitrate (electron acceptor), where nitrate is then possibly reduced via the DNRA pathway (Guven et al., 2005; Kartal et al., 2007a, 2007b). Based on d13C values of lipids and substrates, it was speculated that acetate was not directly incorporated into the biomass, but first degraded into CO2 and then fixed via the acetyl-CoA pathway (Kartal et al., 2008). In fact, it is reported that different anammox consortia may behave discrepantly under acetate stress (Huang et al., 2014; Kartal et al., 2008). Therefore, it may be helpful to explore potential discrepancies in gene expression among different anammox species during the acetate oxidation process. Based on metagenomics and metatranscriptomics analysis, we discovered three distinct routes for acetate transformation in anammox bacteria. Considering that anammox bacteria had the highest abundance and highest expression levels of genes related with acetate metabolism (Fig. 3 and Supplementary Data), the acetate was mainly metabolized by anammox bacteria rather than denitrifying bacteria. Compared to J. caeni, B. sinica was more capable of acetate and acetyl-CoA transformation, and acetate metabolic pathways of B. sinica were more versatile, conferring a competitive advantage to B. sinica in a mixotrophic environment. Acetyl-CoA pathway is a common route for acetate transformation in bacteria (Krivoruchko et al., 2015). Both B. sinica and J. caeni possess AMP-Acs and ADP-Acs. Both these enzymes are capable of catalyzing acetate into acetyl-CoA, but via different mechanisms, as ADP-Acs catalyzes the synthesis of acetyl-CoA from acetate in a single step, while AMP-Acs synthesizes it in two steps (Starai and Escalante-Semerena, 2004). And the AMP-Acs route is a high af- finity pathway, as the Km for acetate was 200 mM and the reaction occurs at a low acetate concentration (Krivoruchko et al., 2015). Discrepancies in AMP-acs between the two anammox bacteria may be one significant reason for their response to acetate being Fig. 4. Expression profile of genes involved in amino acids and B-vitamins cross-feedings in anammox consortia. Relative gene expression of (a) amino acid biosynthetic pathways, (b) amino acid degradation pathways and (c) vitamin B biosynthetic pathways in each species. Colour intensity represents log2 transformed gene expression level, which was obtained by median TPM values across each pathway. One black dot represents p < 0.05 by t-test, and two dots represents p < 0.01 by t-test. (d) Peptidases possibly involved in extracellular protein degradation of each species in autotrophic and mixotrophic anammox consortia. The location of peptidase was predicted using the subcellular localization predictor (CELLO). (e) Amino acid and oligopeptide transporters of each species in autotrophic and mixotrophic anammox consortia. Count number was represented by bubble diameter, and gene expression was represented by bubble colour intensity. * represents p < 0.05 by two-tail t-test, ** represents p < 0.01 by two-tail t-test. AMX1 and AMX2 represents J. caeni and B. sinica, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.) Y. Feng et al. / Water Research 165 (2019) 114974 7