正在加载图片...
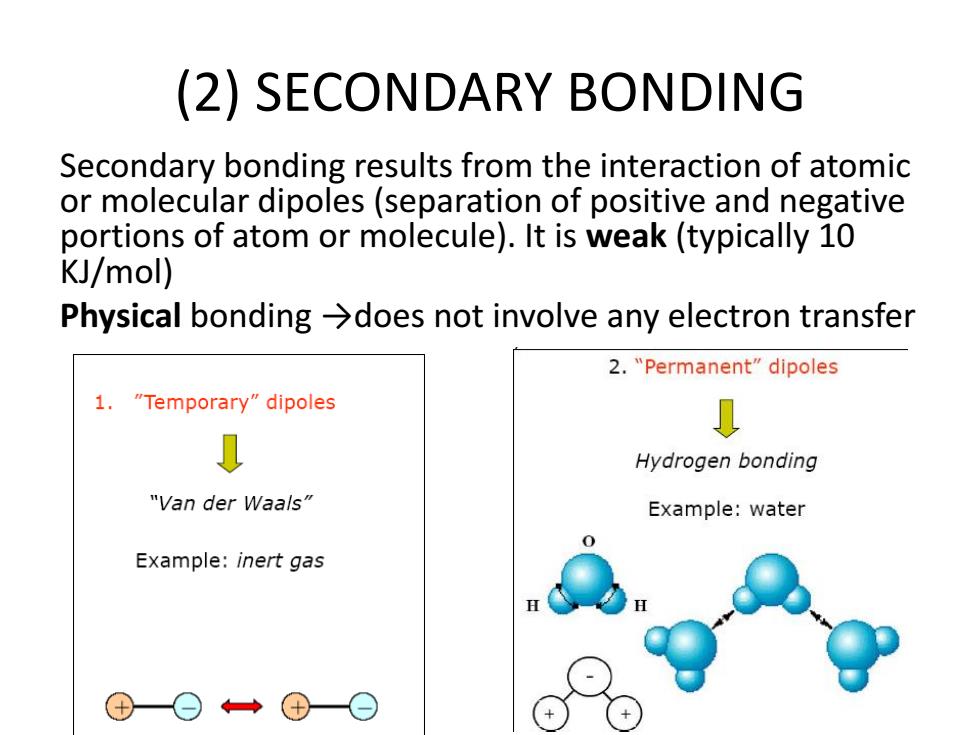
(2)SECONDARY BONDING Secondary bonding results from the interaction of atomic or molecular dipoles(separation of positive and negative portions of atom or molecule).It is weak(typically 10 KJ/mol) Physical bonding >does not involve any electron transfer 2."Permanent"dipoles 1."Temporary"dipoles Hydrogen bonding "Van der Waals" Example:water 0 Example:inert gas(2) SECONDARY BONDING Secondary bonding results from the interaction of atomic or molecular dipoles (separation of positive and negative portions of atom or molecule). It is weak (typically 10 KJ/mol) Physical bonding →does not involve any electron transfer