正在加载图片...
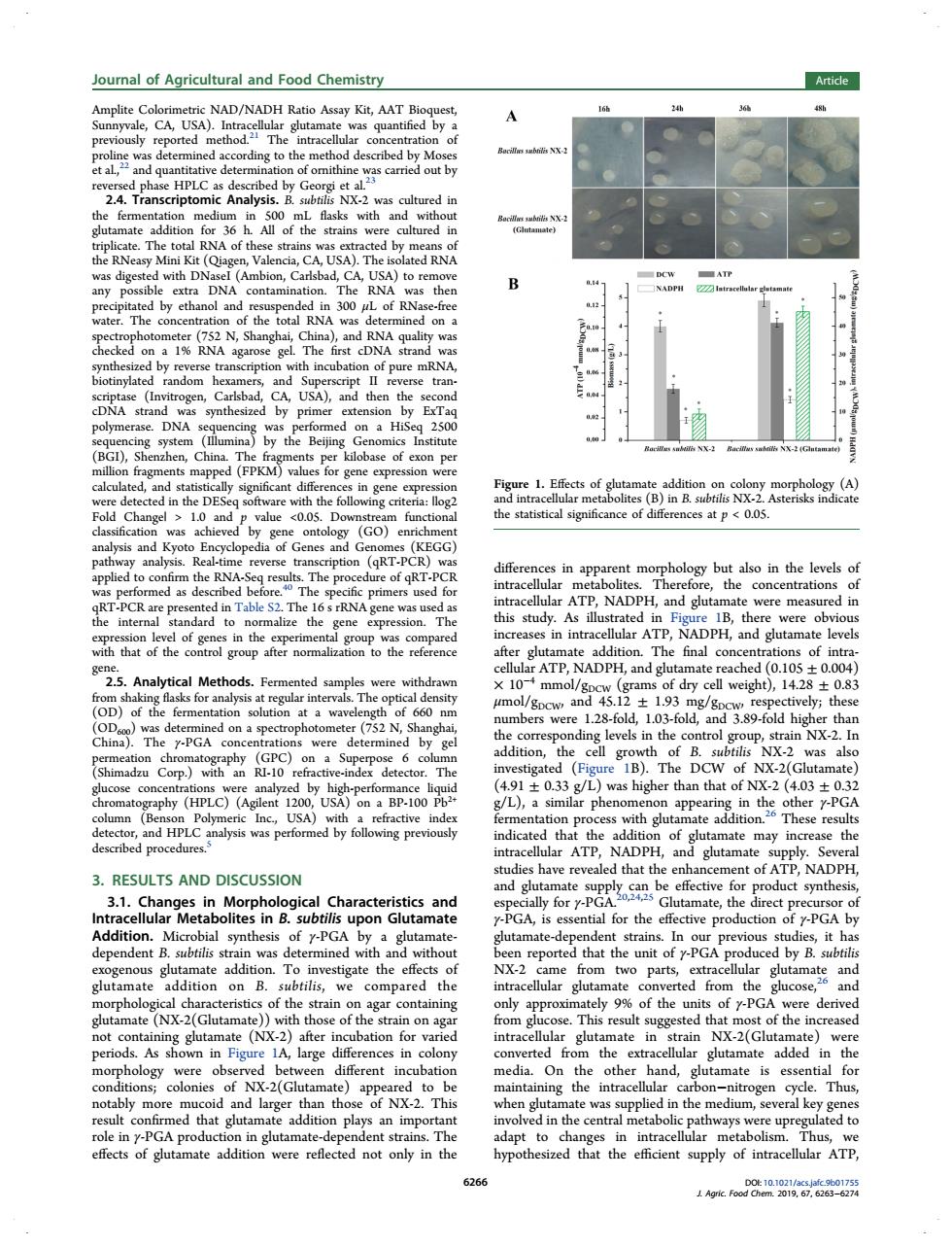
Journal of Agricultural and Food Chemistry Article Amplite Col nyvale,CA, NAD/NADH quanti g to the the stral e total rna o a by AUSA)T d with D bion. )to DN RN the 30 otinythte random DNA (FPKM) and DE ficant dif at p (GOY Ency RNA apparent morpnology ned as PCR RN h the in intra ular ATP DPH, and g of the 2.5.Anab ATP.NADPH,and nat ached (0.105 D)of the n4.12±1.93 ver 3.89-fold y-PGA B).T aphy (HPLC)( 491+03 L)was than that of NX-2 (403+037 VL) ar pher ith appearing in the other y as perfo ng pr of gut ate may 3.RESULTS AND DISCUSSION adies aled that the en nt of ATP.NADPH and P it hat nit fy-PGAPo by B. glutamate addition on B. btilis,we compar ate red the in ted from the and NX-2(Gl of y-P de ate (NX- after ubation for v train NX. (Glut obs differ ent in batio the othe hand. tamate is ess id nd hat gluta ddition plays mp yed to mateaddition were reflected not only in the ed that the Amplite Colorimetric NAD/NADH Ratio Assay Kit, AAT Bioquest, Sunnyvale, CA, USA). Intracellular glutamate was quantified by a previously reported method.21 The intracellular concentration of proline was determined according to the method described by Moses et al.,22 and quantitative determination of ornithine was carried out by reversed phase HPLC as described by Georgi et al.23 2.4. Transcriptomic Analysis. B. subtilis NX-2 was cultured in the fermentation medium in 500 mL flasks with and without glutamate addition for 36 h. All of the strains were cultured in triplicate. The total RNA of these strains was extracted by means of the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). The isolated RNA was digested with DNaseI (Ambion, Carlsbad, CA, USA) to remove any possible extra DNA contamination. The RNA was then precipitated by ethanol and resuspended in 300 μL of RNase-free water. The concentration of the total RNA was determined on a spectrophotometer (752 N, Shanghai, China), and RNA quality was checked on a 1% RNA agarose gel. The first cDNA strand was synthesized by reverse transcription with incubation of pure mRNA, biotinylated random hexamers, and Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA, USA), and then the second cDNA strand was synthesized by primer extension by ExTaq polymerase. DNA sequencing was performed on a HiSeq 2500 sequencing system (Illumina) by the Beijing Genomics Institute (BGI), Shenzhen, China. The fragments per kilobase of exon per million fragments mapped (FPKM) values for gene expression were calculated, and statistically significant differences in gene expression were detected in the DESeq software with the following criteria: |log2 Fold Change| > 1.0 and p value <0.05. Downstream functional classification was achieved by gene ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis. Real-time reverse transcription (qRT-PCR) was applied to confirm the RNA-Seq results. The procedure of qRT-PCR was performed as described before.40 The specific primers used for qRT-PCR are presented in Table S2. The 16 s rRNA gene was used as the internal standard to normalize the gene expression. The expression level of genes in the experimental group was compared with that of the control group after normalization to the reference gene. 2.5. Analytical Methods. Fermented samples were withdrawn from shaking flasks for analysis at regular intervals. The optical density (OD) of the fermentation solution at a wavelength of 660 nm (OD600) was determined on a spectrophotometer (752 N, Shanghai, China). The γ-PGA concentrations were determined by gel permeation chromatography (GPC) on a Superpose 6 column (Shimadzu Corp.) with an RI-10 refractive-index detector. The glucose concentrations were analyzed by high-performance liquid chromatography (HPLC) (Agilent 1200, USA) on a BP-100 Pb2+ column (Benson Polymeric Inc., USA) with a refractive index detector, and HPLC analysis was performed by following previously described procedures.5 3. RESULTS AND DISCUSSION 3.1. Changes in Morphological Characteristics and Intracellular Metabolites in B. subtilis upon Glutamate Addition. Microbial synthesis of γ-PGA by a glutamatedependent B. subtilis strain was determined with and without exogenous glutamate addition. To investigate the effects of glutamate addition on B. subtilis, we compared the morphological characteristics of the strain on agar containing glutamate (NX-2(Glutamate)) with those of the strain on agar not containing glutamate (NX-2) after incubation for varied periods. As shown in Figure 1A, large differences in colony morphology were observed between different incubation conditions; colonies of NX-2(Glutamate) appeared to be notably more mucoid and larger than those of NX-2. This result confirmed that glutamate addition plays an important role in γ-PGA production in glutamate-dependent strains. The effects of glutamate addition were reflected not only in the differences in apparent morphology but also in the levels of intracellular metabolites. Therefore, the concentrations of intracellular ATP, NADPH, and glutamate were measured in this study. As illustrated in Figure 1B, there were obvious increases in intracellular ATP, NADPH, and glutamate levels after glutamate addition. The final concentrations of intracellular ATP, NADPH, and glutamate reached (0.105 ± 0.004) × 10−4 mmol/gDCW (grams of dry cell weight), 14.28 ± 0.83 μmol/gDCW, and 45.12 ± 1.93 mg/gDCW, respectively; these numbers were 1.28-fold, 1.03-fold, and 3.89-fold higher than the corresponding levels in the control group, strain NX-2. In addition, the cell growth of B. subtilis NX-2 was also investigated (Figure 1B). The DCW of NX-2(Glutamate) (4.91 ± 0.33 g/L) was higher than that of NX-2 (4.03 ± 0.32 g/L), a similar phenomenon appearing in the other γ-PGA fermentation process with glutamate addition.26 These results indicated that the addition of glutamate may increase the intracellular ATP, NADPH, and glutamate supply. Several studies have revealed that the enhancement of ATP, NADPH, and glutamate supply can be effective for product synthesis, especially for γ-PGA.20,24,25 Glutamate, the direct precursor of γ-PGA, is essential for the effective production of γ-PGA by glutamate-dependent strains. In our previous studies, it has been reported that the unit of γ-PGA produced by B. subtilis NX-2 came from two parts, extracellular glutamate and intracellular glutamate converted from the glucose,26 and only approximately 9% of the units of γ-PGA were derived from glucose. This result suggested that most of the increased intracellular glutamate in strain NX-2(Glutamate) were converted from the extracellular glutamate added in the media. On the other hand, glutamate is essential for maintaining the intracellular carbon−nitrogen cycle. Thus, when glutamate was supplied in the medium, several key genes involved in the central metabolic pathways were upregulated to adapt to changes in intracellular metabolism. Thus, we hypothesized that the efficient supply of intracellular ATP, Figure 1. Effects of glutamate addition on colony morphology (A) and intracellular metabolites (B) in B. subtilis NX-2. Asterisks indicate the statistical significance of differences at p < 0.05. Journal of Agricultural and Food Chemistry Article DOI: 10.1021/acs.jafc.9b01755 J. Agric. Food Chem. 2019, 67, 6263−6274 6266