正在加载图片...
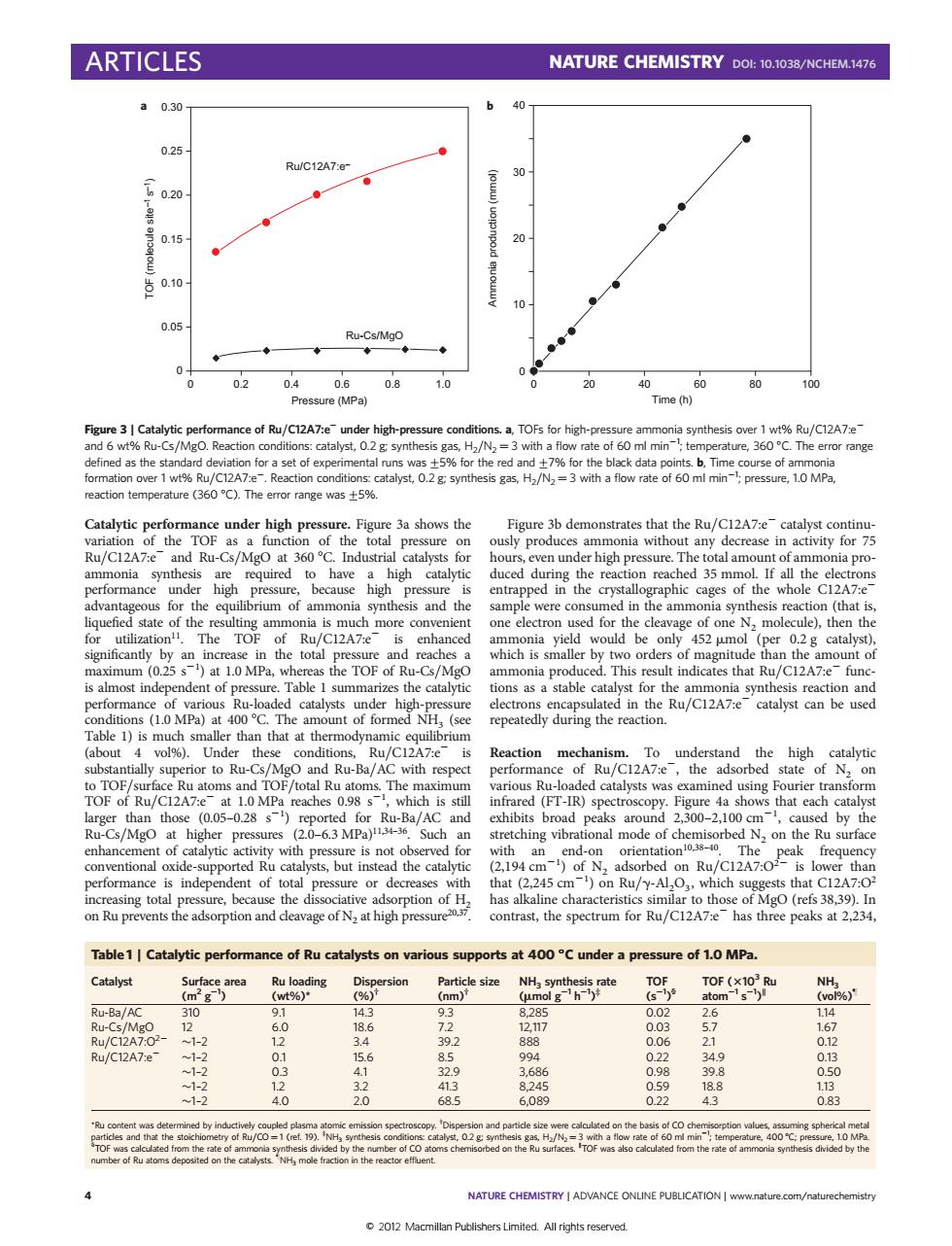
ARTICLES NATURE CHEMISTRY DOI:10.1038/NCHEM.1476 a0.30 0.25 Ru/C12A7: 30 02 0 0 0204 0.6 08 20 % 60 essure (MPa e (h Ru/C12A7:e +9%R/C12A7 talyst.0.2 g .H/N=3 e of 60 m min ure,360C.The rang d as the 1wt%Ru/C12A7 o (360C).The emror range was% s that the Ru/C12A7-e- Catav the Tor nia withou quired to have a duced dur ing the re ioreached 35 mm a p us for the e ilibrium of an synthesis and the reaction (that i en th which is 12A7 is almost indepe ntof-ade Table I summ the catalyt unt of for vol%) Und the Ru/C1 A7- Reaction mechanism.To understand the high nd TOF/total Ru The arious Ru-l Fourier transt s/MgO higher P tr ing vibr ol mode of ch N2 on the hat (2.245 of rbe on Ru/C ng to a alkali Table1 Catalytic performance of Ru catalysts on various supports at 400"C under a pressure of 1.0 MPa. Catalyst area Particle size TOF (0 RU/C12A7 oo 49 08 NATURE CHEMISTRY ADVANCE ONLINE PUBLICATION wwwnature.com/naturechemistr 2012 Macmillan Publishers Limited All rights reserved. Catalytic performance under high pressure. Figure 3a shows the variation of the TOF as a function of the total pressure on Ru/C12A7:e2 and Ru-Cs/MgO at 360 8C. Industrial catalysts for ammonia synthesis are required to have a high catalytic performance under high pressure, because high pressure is advantageous for the equilibrium of ammonia synthesis and the liquefied state of the resulting ammonia is much more convenient for utilization11. The TOF of Ru/C12A7:e2 is enhanced significantly by an increase in the total pressure and reaches a maximum (0.25 s21 ) at 1.0 MPa, whereas the TOF of Ru-Cs/MgO is almost independent of pressure. Table 1 summarizes the catalytic performance of various Ru-loaded catalysts under high-pressure conditions (1.0 MPa) at 400 8C. The amount of formed NH3 (see Table 1) is much smaller than that at thermodynamic equilibrium (about 4 vol%). Under these conditions, Ru/C12A7:e2 is substantially superior to Ru-Cs/MgO and Ru-Ba/AC with respect to TOF/surface Ru atoms and TOF/total Ru atoms. The maximum TOF of Ru/C12A7:e2 at 1.0 MPa reaches 0.98 s21 , which is still larger than those (0.05–0.28 s21 ) reported for Ru-Ba/AC and Ru-Cs/MgO at higher pressures (2.0–6.3 MPa)11,34–36. Such an enhancement of catalytic activity with pressure is not observed for conventional oxide-supported Ru catalysts, but instead the catalytic performance is independent of total pressure or decreases with increasing total pressure, because the dissociative adsorption of H2 on Ru prevents the adsorption and cleavage of N2 at high pressure20,37. Figure 3b demonstrates that the Ru/C12A7:e2 catalyst continuously produces ammonia without any decrease in activity for 75 hours, even under high pressure. The total amount of ammonia produced during the reaction reached 35 mmol. If all the electrons entrapped in the crystallographic cages of the whole C12A7:e2 sample were consumed in the ammonia synthesis reaction (that is, one electron used for the cleavage of one N2 molecule), then the ammonia yield would be only 452 mmol (per 0.2 g catalyst), which is smaller by two orders of magnitude than the amount of ammonia produced. This result indicates that Ru/C12A7:e2 functions as a stable catalyst for the ammonia synthesis reaction and electrons encapsulated in the Ru/C12A7:e2 catalyst can be used repeatedly during the reaction. Reaction mechanism. To understand the high catalytic performance of Ru/C12A7:e2, the adsorbed state of N2 on various Ru-loaded catalysts was examined using Fourier transform infrared (FT-IR) spectroscopy. Figure 4a shows that each catalyst exhibits broad peaks around 2,300–2,100 cm21 , caused by the stretching vibrational mode of chemisorbed N2 on the Ru surface with an end-on orientation10,38–40. The peak frequency (2,194 cm21 ) of N2 adsorbed on Ru/C12A7:O22 is lower than that (2,245 cm21 ) on Ru/g-Al2O3, which suggests that C12A7:O2 has alkaline characteristics similar to those of MgO (refs 38,39). In contrast, the spectrum for Ru/C12A7:e2 has three peaks at 2,234, Pressure (MPa) 0 0.2 0.4 0.6 0.8 1.0 0 0.30 0.25 0.20 0.15 0.10 0.05 a TOF (molecule site–1 s–1) Time (h) Ammonia production (mmol) 40 30 20 10 0 0 20 40 60 80 100 b Ru/C12A7:e– Ru-Cs/MgO Figure 3 | Catalytic performance of Ru/C12A7:e2 under high-pressure conditions. a, TOFs for high-pressure ammonia synthesis over 1 wt% Ru/C12A7:e2 and 6 wt% Ru-Cs/MgO. Reaction conditions: catalyst, 0.2 g; synthesis gas, H2/N2 ¼ 3 with a flow rate of 60 ml min21 ; temperature, 360 8C. The error range defined as the standard deviation for a set of experimental runs was+5% for the red and+7% for the black data points. b, Time course of ammonia formation over 1 wt% Ru/C12A7:e2. Reaction conditions: catalyst, 0.2 g; synthesis gas, H2/N2 ¼ 3 with a flow rate of 60 ml min21 ; pressure, 1.0 MPa, reaction temperature (360 8C). The error range was+5%. Table 1 | Catalytic performance of Ru catalysts on various supports at 400 8C under a pressure of 1.0 MPa. Catalyst Surface area (m2 g21 ) Ru loading (wt%)* Dispersion (%)† Particle size (nm)† NH3 synthesis rate (mmol g21 h21 ) ‡ TOF (s21 ) § TOF (3103 Ru atom21 s 21 ) NH3 (vol%)} Ru-Ba/AC 310 9.1 14.3 9.3 8,285 0.02 2.6 1.14 Ru-Cs/MgO 12 6.0 18.6 7.2 12,117 0.03 5.7 1.67 Ru/C12A7:O22 1–2 1.2 3.4 39.2 888 0.06 2.1 0.12 Ru/C12A7:e2 1–2 0.1 15.6 8.5 994 0.22 34.9 0.13 1–2 0.3 4.1 32.9 3,686 0.98 39.8 0.50 1–2 1.2 3.2 41.3 8,245 0.59 18.8 1.13 1–2 4.0 2.0 68.5 6,089 0.22 4.3 0.83 *Ru content was determined by inductively coupled plasma atomic emission spectroscopy. † Dispersion and particle size were calculated on the basis of CO chemisorption values, assuming spherical metal particles and that the stoichiometry of Ru/CO ¼ 1 (ref. 19). ‡ NH3 synthesis conditions: catalyst, 0.2 g; synthesis gas, H2/N2 ¼ 3 with a flow rate of 60 ml min21 ; temperature, 400 8C; pressure, 1.0 MPa. § TOF was calculated from the rate of ammonia synthesis divided by the number of CO atoms chemisorbed on the Ru surfaces. TOF was also calculated from the rate of ammonia synthesis divided by the number of Ru atoms deposited on the catalysts. } NH3 mole fraction in the reactor effluent. ARTICLES NATURE CHEMISTRY DOI: 10.1038/NCHEM.1476 4 NATURE CHEMISTRY | ADVANCE ONLINE PUBLICATION | www.nature.com/naturechemistry © 2012 Macmillan Publishers Limited. All rights reserved.�������