正在加载图片...
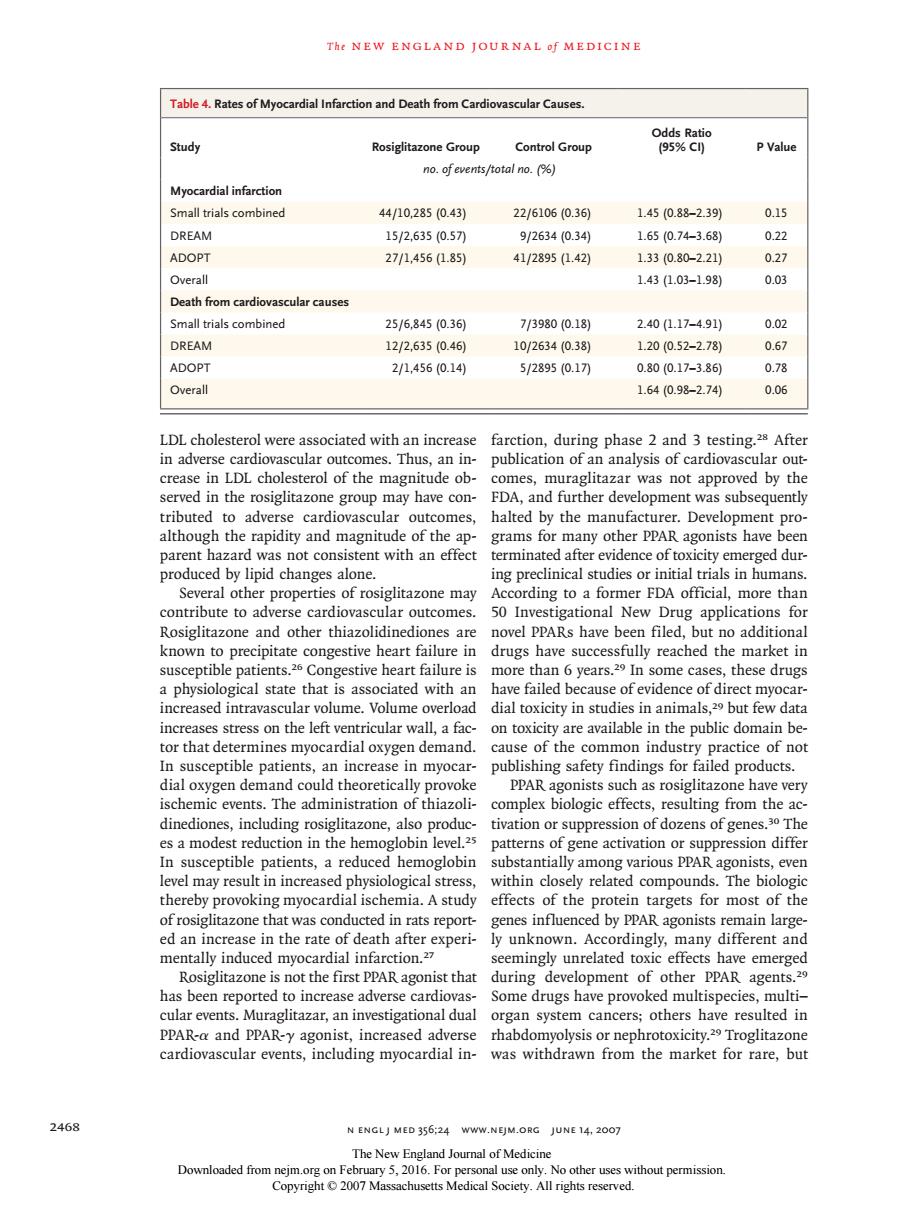
The NEW ENGLAND JOURNAL Of MEDICINE Table 4.Rates of Myo rdial Infarction and Death from Cardiovascular Causes Study Control Group PValu no.ofevents/total no.() Myocardial infarctior Small trials combined 44/10,2850.43) 22/61060.36) 1.450.88-2.391 0.15 DREAM 15/2,6350.5刀 9/26340.340 1.650.74-3.68 022 ADOPT 271,456.85 41/28951.42) 1.330.80-2.210 0.27 143L03-1.98 0.03 h from cardiovascular cause all trials combine 25/6,8450.36 7/3980(0.18 2.40(17-491 DREAM 12/2.6350.46 10/2634(0.38 1.200.52-2.78) 0.67 ADOPT 2/1.4560.14) 5/289510.17刀 0.8010.17-3.86 0.78 Overall 1.640.98-2.74 0.06 LDLcholesterol were associated with an increase farction,during phase 2 and 3 testing.After in adverse cardic nes.Thus,an in- publication of an analysis of cardiovascular out ster wve con opm nitude of y the ma nent pro parent hazard was not consistent with an effect terminated after evidence of toxiciry emerg ed dur produced by lipid changes alone. ing preclinical studies or initial trials in humans Several other properties of rosiglitazone may According to a former FDA official,more thar contribute to adverse cardi ovascular outcomes 50 Investigational New rug applications fo novel PPARs have ut no additiona known precip ure ly reachec the mar a physiological stare thats ciated with ar increased intravascular volume volume overload dial toxicity in studies in animals 29 but few data increases stress on the left ventricular wall,a fac on toxicity are available in the public domain be tor that determines myocardial oxygen demand. cause of the common industry practice of no In susceptib e patients, increas publishing safety findings for ailed pro ducts oxygen de zone na P es a modest reductio in the he In susceptible patients,a reduced hemoglobin substantially among various PPAR agonists,even level may result in increased physiological stress within closely related compounds.The biologic thereby provoking myocardial ischemia.A study effects of the protein targets for most of the of rosiglitazone th was cond ed in rats repor d by PPAR agonists remain large ed an i rate c ind d nt and during of other ents has been reported to increase adverse cardiovas Some drugs have provoked multispecies multi- cular events.Muraglitazar,an investigational dual organ system cancers;others have resulted in PPAR-a and PPAR-y agonist,increased adverse rhabdomyolysis or nephrotoxicity.Troglitazone cardiovascular events,including myocardial in- was withdrawn from the market for rare,but 2468 N ENGLJ MED 356:24 WWW.NEJM.ORG JUNE 14.2007 Downloaded from 5,2016.F 15T h e n e w e ng l a nd j o u r na l o f m e dic i n e 2468 n engl j med 356;24 www.nejm.org june 14, 2007 LDL cholesterol were associated with an increase in adverse cardiovascular outcomes. Thus, an increase in LDL cholesterol of the magnitude observed in the rosiglitazone group may have contributed to adverse cardiovascular outcomes, although the rapidity and magnitude of the apparent hazard was not consistent with an effect produced by lipid changes alone. Several other properties of rosiglitazone may contribute to adverse cardiovascular outcomes. Rosiglitazone and other thiazolidinediones are known to precipitate congestive heart failure in susceptible patients.26 Congestive heart failure is a physiological state that is associated with an increased intravascular volume. Volume overload increases stress on the left ventricular wall, a factor that determines myocardial oxygen demand. In susceptible patients, an increase in myocardial oxygen demand could theoretically provoke ischemic events. The administration of thiazolidinediones, including rosiglitazone, also produces a modest reduction in the hemoglobin level.25 In susceptible patients, a reduced hemoglobin level may result in increased physiological stress, thereby provoking myocardial ischemia. A study of rosiglitazone that was conducted in rats reported an increase in the rate of death after experimentally induced myocardial infarction.27 Rosiglitazone is not the first PPAR agonist that has been reported to increase adverse cardiovascular events. Muraglitazar, an investigational dual PPAR-α and PPAR-γ agonist, increased adverse cardiovascular events, including myocardial infarction, during phase 2 and 3 testing.28 After publication of an analysis of cardiovascular outcomes, muraglitazar was not approved by the FDA, and further development was subsequently halted by the manufacturer. Development programs for many other PPAR agonists have been terminated after evidence of toxicity emerged during preclinical studies or initial trials in humans. According to a former FDA official, more than 50 Investigational New Drug applications for novel PPARs have been filed, but no additional drugs have successfully reached the market in more than 6 years.29 In some cases, these drugs have failed because of evidence of direct myocardial toxicity in studies in animals,29 but few data on toxicity are available in the public domain because of the common industry practice of not publishing safety findings for failed products. PPAR agonists such as rosiglitazone have very complex biologic effects, resulting from the activation or suppression of dozens of genes.30 The patterns of gene activation or suppression differ substantially among various PPAR agonists, even within closely related compounds. The biologic effects of the protein targets for most of the genes influenced by PPAR agonists remain largely unknown. Accordingly, many different and seemingly unrelated toxic effects have emerged during development of other PPAR agents.29 Some drugs have provoked multispecies, multi– organ system cancers; others have resulted in rhabdomyolysis or nephrotoxicity.29 Troglitazone was withdrawn from the market for rare, but Table 4. Rates of Myocardial Infarction and Death from Cardiovascular Causes. Study Rosiglitazone Group Control Group Odds Ratio (95% CI) P Value no. of events/total no. (%) Myocardial infarction Small trials combined 44/10,285 (0.43) 22/6106 (0.36) 1.45 (0.88–2.39) 0.15 DREAM 15/2,635 (0.57) 9/2634 (0.34) 1.65 (0.74–3.68) 0.22 ADOPT 27/1,456 (1.85) 41/2895 (1.42) 1.33 (0.80–2.21) 0.27 Overall 1.43 (1.03–1.98) 0.03 Death from cardiovascular causes Small trials combined 25/6,845 (0.36) 7/3980 (0.18) 2.40 (1.17–4.91) 0.02 DREAM 12/2,635 (0.46) 10/2634 (0.38) 1.20 (0.52–2.78) 0.67 ADOPT 2/1,456 (0.14) 5/2895 (0.17) 0.80 (0.17–3.86) 0.78 Overall 1.64 (0.98–2.74) 0.06 The New England Journal of Medicine Downloaded from nejm.org on February 5, 2016. For personal use only. No other uses without permission. Copyright © 2007 Massachusetts Medical Society. All rights reserved