正在加载图片...
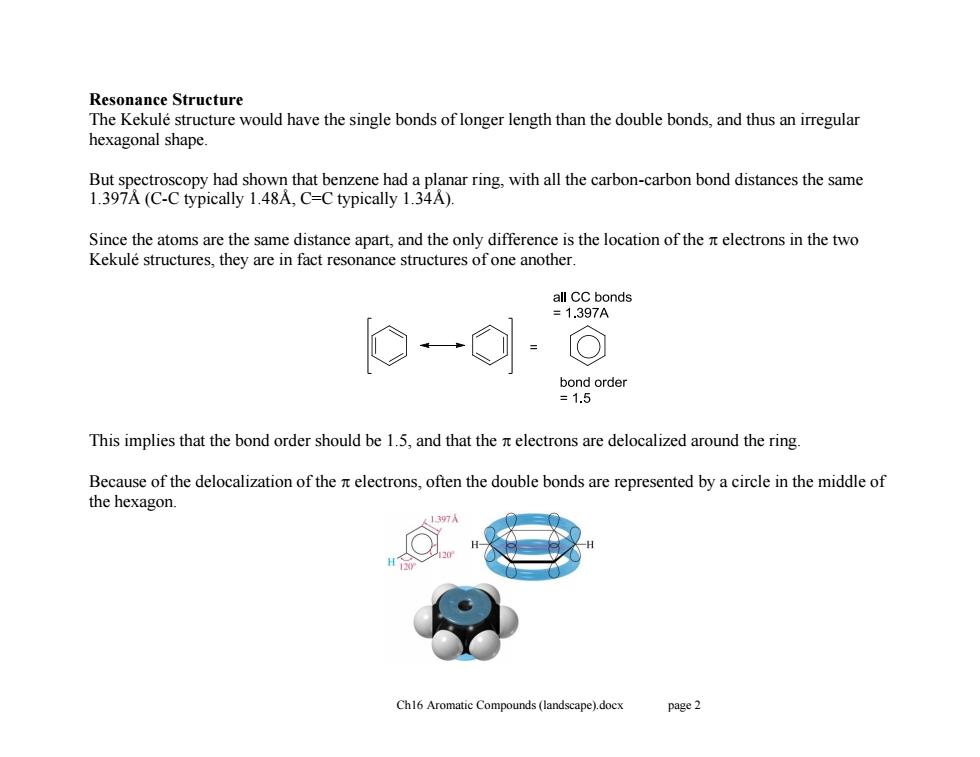
Resonance Structure The Kekule structure would have the single bonds of longer length than the double bonds,and thus an irregular hexagonal shape. But spectroscopy had shown that benzene had a planar ring,with all the carbon-carbon bond distances the same 1.397A(C-C typically 1.48A,C=C typically 1.34A). Since the atoms are the same distance apart,and the only difference is the location of the electrons in the two Kekule structures,they are in fact resonance structures of one another all CC bonds =1.397A bond order =1.5 This implies that the bond order should be 1.5,and that the nelectrons are delocalized around the ring. Because of the delocalization of the electrons,often the double bonds are represented by a circle in the middle of the hexagon. Ch16 Aromatic Compounds (landscape).docx page 2Ch16 Aromatic Compounds (landscape).docx page 2 Resonance Structure The Kekulé structure would have the single bonds of longer length than the double bonds, and thus an irregular hexagonal shape. But spectroscopy had shown that benzene had a planar ring, with all the carbon-carbon bond distances the same 1.397Å (C-C typically 1.48Å, C=C typically 1.34Å). Since the atoms are the same distance apart, and the only difference is the location of the electrons in the two Kekulé structures, they are in fact resonance structures of one another. This implies that the bond order should be 1.5, and that the electrons are delocalized around the ring. Because of the delocalization of the electrons, often the double bonds are represented by a circle in the middle of the hexagon