正在加载图片...
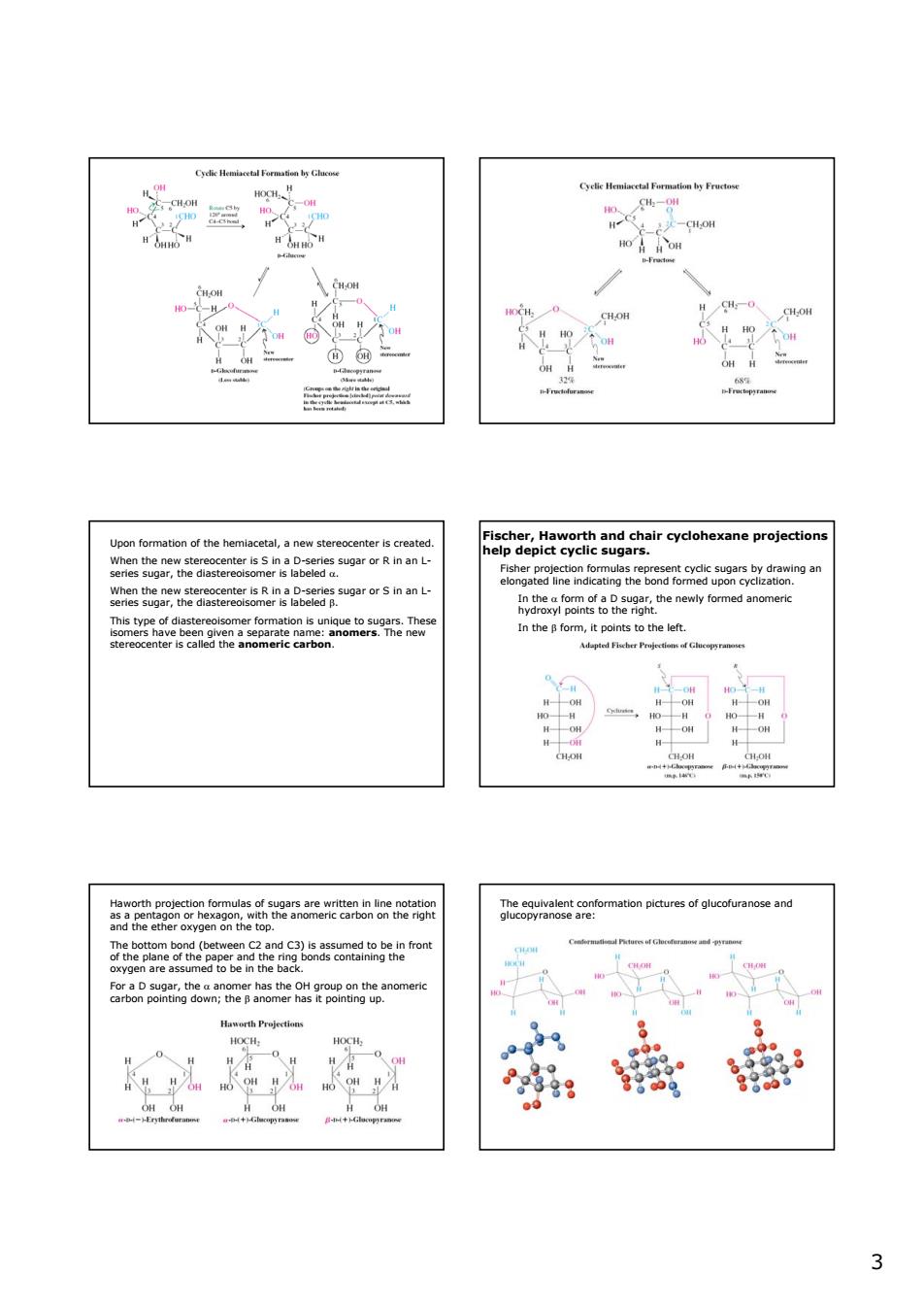
温 -C0 0 Fis ethesetteetrtesRnBeatugerorsnan n,p to the le解 2e5aeehn6 eoguetneomatonpctunsofglucahtanoseand c子5om graaomng6tnernsmesgepe8ngeanomen 33 Upon formation of the hemiacetal, a new stereocenter is created. When the new stereocenter is S in a D-series sugar or R in an Lseries sugar, the diastereoisomer is labeled α. When the new stereocenter is R in a D-series sugar or S in an Lseries sugar, the diastereoisomer is labeled β. This type of diastereoisomer formation is unique to sugars. These isomers have been given a separate name: anomers. The new stereocenter is called the anomeric carbon. Fischer, Haworth and chair cyclohexane projections help depict cyclic sugars. Fisher projection formulas represent cyclic sugars by drawing an elongated line indicating the bond formed upon cyclization. In the α form of a D sugar, the newly formed anomeric hydroxyl points to the right. In the β form, it points to the left. Haworth projection formulas of sugars are written in line notation as a pentagon or hexagon, with the anomeric carbon on the right and the ether oxygen on the top. The bottom bond (between C2 and C3) is assumed to be in front of the plane of the paper and the ring bonds containing the oxygen are assumed to be in the back. For a D sugar, the α anomer has the OH group on the anomeric carbon pointing down; the β anomer has it pointing up. The equivalent conformation pictures of glucofuranose and glucopyranose are: