正在加载图片...
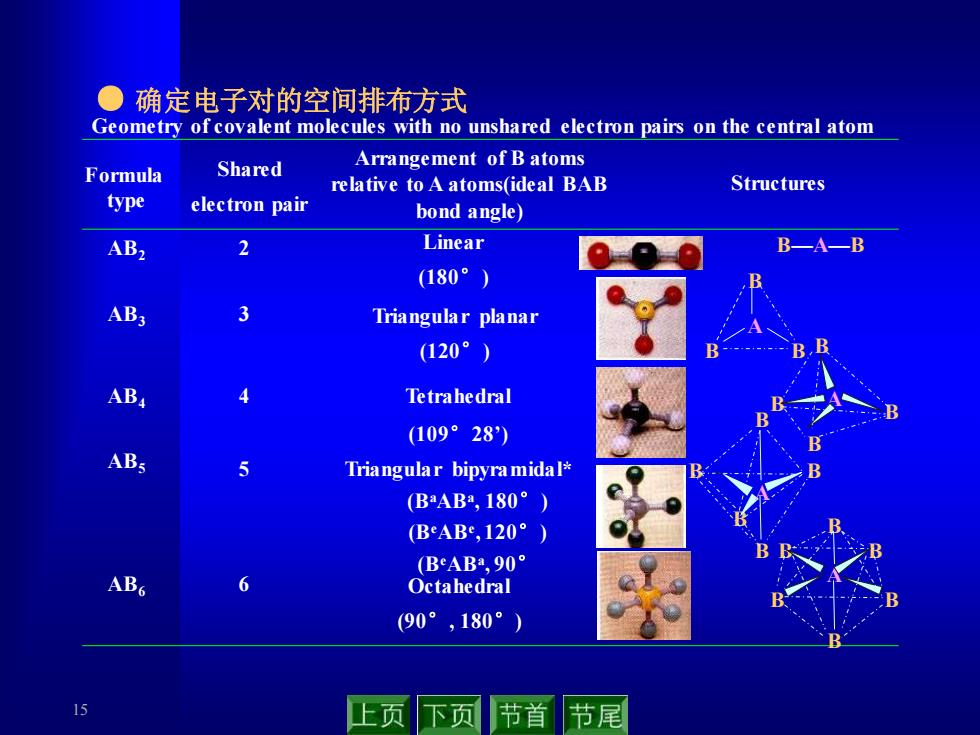
●确定电子对的空间排布方式 Geometry of covalent molecules with no unshared electron pairs on the central atom Shared Arrangement of B atoms Formula relative to A atoms(ideal BAB Structures type electron pair bond angle) AB2 2 Linear BA—B (180°) AB3 3 Triangular planar (120°) B AB4 Tetrahedral (109°28) Triangular bipyramidal* (BAB2,180°) BeAB,120°) (BeAB,90° ABG 6 Octahedral (90°,180°) 15 上页 下页 节首 节尾 15 ● 确定电子对的空间排布方式 Formula type Shared electron pair Arrangement of B atoms relative to A atoms(ideal BAB bond angle) Structures Geometry of covalent molecules with no unshared electron pairs on the central atom 2 Linear (180°) AB B—A—B 2 AB3 3 Triangular planar (120°) A B B B AB4 4 Tetrahedral (109°28’) B A B B B AB5 5 Triangular bipyramidal* (BaABa , 180°) (BeABe , 120°) (BeABa , 90° B B B B B A AB6 6 Octahedral (90°, 180°) A B B B B B B