正在加载图片...
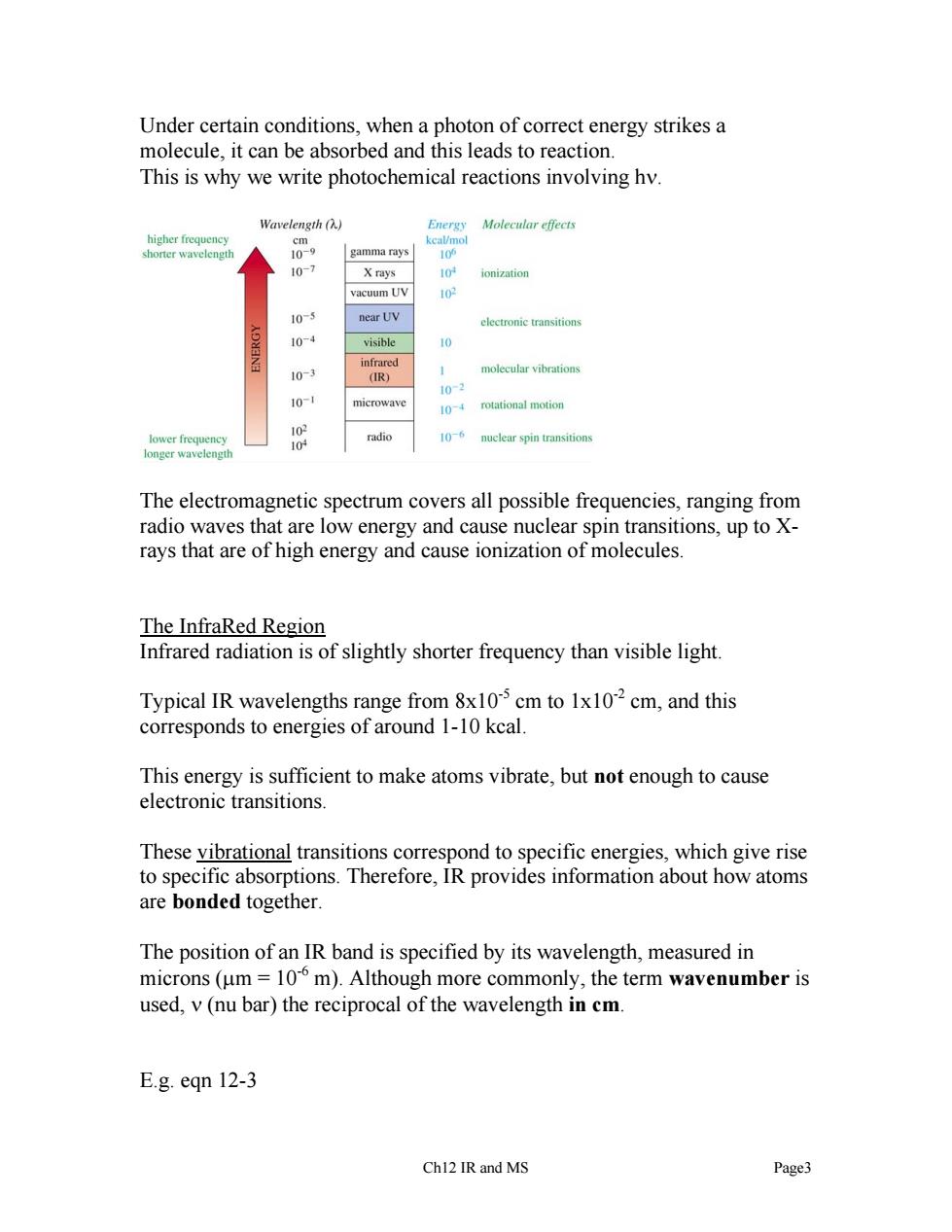
Under certain conditions,when a photon of correct energy strikes a molecule,it can be absorbed and this leads to reaction. This is why we write photochemical reactions involving hv. gth (h.) Moleeular ffeets 109 gamma rays 10-7 ionization electronic transitions visible 10- 0 microwave 1 rotational motion radio 10-6 nuelear spin transitions The electromagnetic spectrum covers all possible frequencies,ranging from radio waves that are low energy and cause nuclear spin transitions,up to X- rays that are of high energy and cause ionization of molecules. The InfraRed Region Infrared radiation is of slightly shorter frequency than visible light Typical IR wavelengths range from 8x10 cm to 1x102cm,and this corresponds to energies of around 1-10 kcal. This energy is sufficient to make atoms vibrate,but not enough to cause electronic transitions. These vibrational transitions correspond to specific energies,which give rise to specific absorptions.Therefore,IR provides information about how atoms are bonded together. The position of an IR band is specified by its wavelength,measured in microns(um=10m).Although more commonly,the term wavenumber is used,v(nu bar)the reciprocal of the wavelength in cm. E.g.eqn 12-3 Chl2 IR and MS Page3Under certain conditions, when a photon of correct energy strikes a molecule, it can be absorbed and this leads to reaction. This is why we write photochemical reactions involving hν. The electromagnetic spectrum covers all possible frequencies, ranging from radio waves that are low energy and cause nuclear spin transitions, up to Xrays that are of high energy and cause ionization of molecules. The InfraRed Region Infrared radiation is of slightly shorter frequency than visible light. Typical IR wavelengths range from 8x10-5 cm to 1x10-2 cm, and this corresponds to energies of around 1-10 kcal. This energy is sufficient to make atoms vibrate, but not enough to cause electronic transitions. These vibrational transitions correspond to specific energies, which give rise to specific absorptions. Therefore, IR provides information about how atoms are bonded together. The position of an IR band is specified by its wavelength, measured in microns (μm = 10-6 m). Although more commonly, the term wavenumber is used, ν (nu bar) the reciprocal of the wavelength in cm. E.g. eqn 12-3 Ch12 IR and MS Page3