正在加载图片...
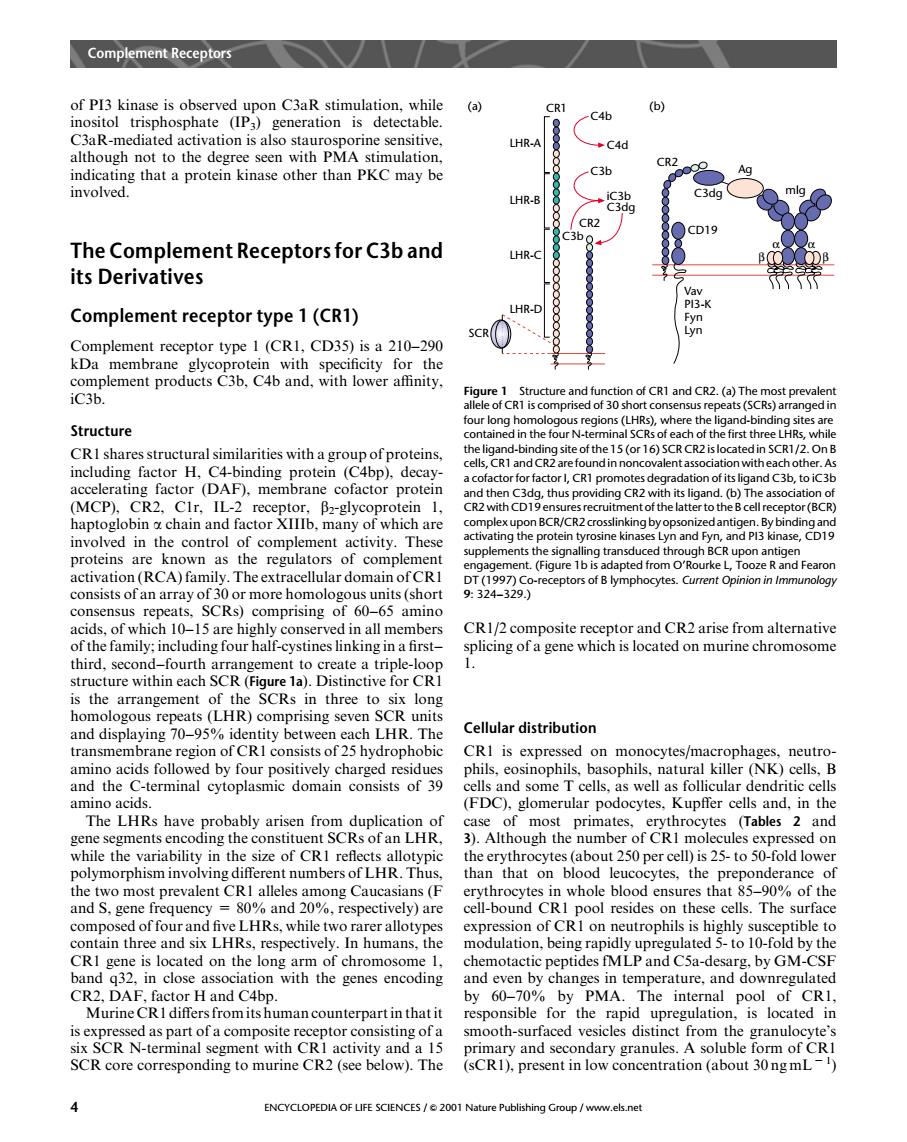
Complemet Reeptor of Pl3 kinase is observed upon C3aR stimulation.while (a) inositol trisphosphate (IP3)generation is detectable. C46 LHR-A C4d ugot than PKC CR2 othe -C3b Ag a prot involved. LHR-B The Complement Receptors for C3b and LHR- its Derivatives Complement receptor type 1(CR1) HR- Complement receptor type 1(CRI,CD35)is a 210-290 kDa membrane Structure our long CRI shares structural similarities witha groupof proteins, (MCP). the thus ling CR2 v g chain ar nd factor Ib plexupon BCR/CR2 crosslinking by ops involved in the control of complement activity.These uppleme proteins are known as the regulators of complemen activation (RCA)family. r domain of C T992329 )comprising of 60-65 a acids,of which 10-15 are highly conserved in all members CR1/composite receptor and CR2 arise from alternative of the family:including four half-cystines linking in a first- splicing of a gene which is located on murine chromosome third.snd CRI the SCRs thr homologous repeats(LHR)comprising seven SCR units and displaying 70- twee LHR The Cellular distribution ra CRI cids foll on n nocytes/ op (NK net onsists of 39 els and some amino acids (FDC).glomerular podocytes.Kupffer cells and.in the The LHRs have probably arisen from duplic mos prin (Tables 2 and hile the en ugn so-tol on than that on blood leuc tes.the pre nderance the two most prevalent CRI alleles among Caucasians(F erythrocytes in whole blood ensures that 85-90%of the and S.gene 0%and 20%.respectively)are cell-bound CRI pool resides on these cells.I he surface compos of fou Iks.while two n th is located on the long arm of chr ptides fMIP and Csa-desa band a32.in close association with the genes encoding and even by changes in temperature,and downregulated H and C4bp. by PMA.The internal pool of CRI an counterpa he rapic SCR N-terminal and a 15 nary and se SCR core corresponding to murine CR2(see below).The (sCR).present in low concentration (about 30ngmL) ENCYCLOPEDIA OF LIFE SCIENCES/e2001 Na v.els netof PI3 kinase is observed upon C3aR stimulation, while inositol trisphosphate (IP3) generation is detectable. C3aR-mediated activation is also staurosporine sensitive, although not to the degree seen with PMA stimulation, indicating that a protein kinase other than PKCmay be involved. The Complement Receptors for C3b and its Derivatives Complement receptor type 1 (CR1) Complement receptor type 1 (CR1, CD35) is a 210–290 kDa membrane glycoprotein with specificity for the complement products C3b, C4b and, with lower affinity, iC3b. Structure CR1 shares structural similarities with a group of proteins, including factor H, C4-binding protein (C4bp), decayaccelerating factor (DAF), membrane cofactor protein (MCP), CR2, C1r, IL-2 receptor, b2-glycoprotein 1, haptoglobin a chain and factor XIIIb, many of which are involved in the control of complement activity. These proteins are known as the regulators of complement activation (RCA) family. The extracellular domain of CR1 consists of an array of 30 or more homologous units (short consensus repeats, SCRs) comprising of 60–65 amino acids, of which 10–15 are highly conserved in all members of the family; including four half-cystines linking in a first– third, second–fourth arrangement to create a triple-loop structure within each SCR (Figure 1a). Distinctive for CR1 is the arrangement of the SCRs in three to six long homologous repeats (LHR) comprising seven SCR units and displaying 70–95% identity between each LHR. The transmembrane region of CR1 consists of 25 hydrophobic amino acids followed by four positively charged residues and the C-terminal cytoplasmic domain consists of 39 amino acids. The LHRs have probably arisen from duplication of gene segments encoding the constituent SCRs of an LHR, while the variability in the size of CR1 reflects allotypic polymorphism involving different numbers of LHR. Thus, the two most prevalent CR1 alleles among Caucasians (F and S, gene frequency = 80% and 20%, respectively) are composed of four and five LHRs, while two rarer allotypes contain three and six LHRs, respectively. In humans, the CR1 gene is located on the long arm of chromosome 1, band q32, in close association with the genes encoding CR2, DAF, factor H and C4bp. Murine CR1 differs from its human counterpart in that it is expressed as part of a composite receptor consisting of a six SCR N-terminal segment with CR1 activity and a 15 SCR core corresponding to murine CR2 (see below). The CR1/2 composite receptor and CR2 arise from alternative splicing of a gene which is located on murine chromosome 1. Cellular distribution CR1 is expressed on monocytes/macrophages, neutrophils, eosinophils, basophils, natural killer (NK) cells, B cells and some T cells, as well as follicular dendritic cells (FDC), glomerular podocytes, Kupffer cells and, in the case of most primates, erythrocytes (Tables 2 and 3). Although the number of CR1 molecules expressed on the erythrocytes (about 250 per cell) is 25- to 50-fold lower than that on blood leucocytes, the preponderance of erythrocytes in whole blood ensures that 85–90% of the cell-bound CR1 pool resides on these cells. The surface expression of CR1 on neutrophils is highly susceptible to modulation, being rapidly upregulated 5- to 10-fold by the chemotactic peptides fMLP and C5a-desarg, by GM-CSF and even by changes in temperature, and downregulated by 60–70% by PMA. The internal pool of CR1, responsible for the rapid upregulation, is located in smooth-surfaced vesicles distinct from the granulocyte’s primary and secondary granules. A soluble form of CR1 (sCR1), present in low concentration (about 30 ng mL 2 1 ) LHR-A CR1 LHR-B LHR-C LHR-D SCR C4b C4d C3b iC3b C3dg C3b CR2 (a) CR2 (b) CD19 C3dg Ag α α β β mlg Vav PI3-K Fyn Lyn Figure 1 Structure and function of CR1 and CR2. (a) The most prevalent allele of CR1 is comprised of 30 short consensus repeats (SCRs) arranged in four long homologous regions (LHRs), where the ligand-binding sites are contained in the four N-terminal SCRs of each of the first three LHRs, while the ligand-binding site of the 15 (or 16) SCR CR2 is located in SCR1/2. On B cells, CR1 and CR2 are found in noncovalent association with each other. As a cofactor for factor I, CR1 promotes degradation of its ligand C3b, to iC3b and then C3dg, thus providing CR2 with its ligand. (b) The association of CR2 with CD19 ensures recruitment of the latter to the B cell receptor (BCR) complex upon BCR/CR2crosslinking by opsonized antigen. By binding and activating the protein tyrosine kinases Lyn and Fyn, and PI3 kinase, CD19 supplements the signalling transduced through BCR upon antigen engagement. (Figure 1b is adapted from O’Rourke L, Tooze R and Fearon DT (1997) Co-receptors of B lymphocytes. Current Opinion in Immunology 9: 324–329.) Complement Receptors 4 ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net