正在加载图片...
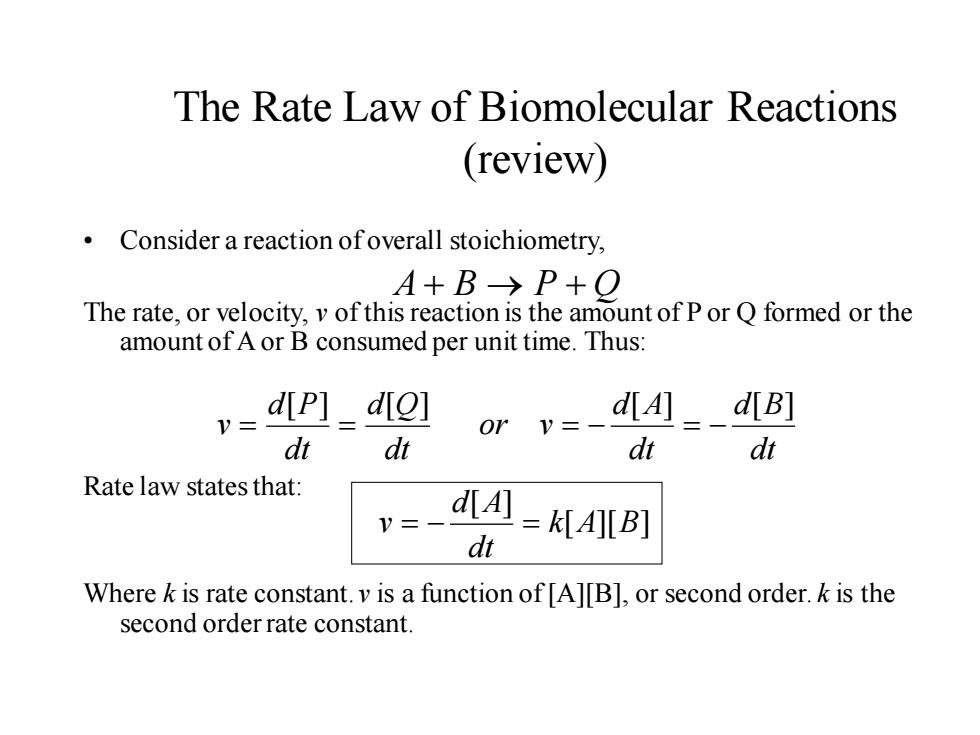
The Rate Law of Biomolecular Reactions (review) Consider a reaction ofoverall stoichiometry, A+B→P+O The rate,or velocity,v of this reaction is the amount of P or Q formed or the amount of A or B consumed per unit time.Thus: dip]_diol dt dt or v=- d[A]d[B] dt dt Rate law states that: d[A =KIAl[B] dt Where k is rate constant.v is a function of [A][B],or second order.k is the second order rate constant.The Rate Law of Biomolecular Reactions (review) • Consider a reaction of overall stoichiometry, The rate, or velocity, v of this reaction is the amount of P or Q formed or the amount of A or B consumed per unit time. Thus: Rate law states that: Where k is rate constant. v is a function of [A][B], or second order. k is the second order rate constant. dt d B dt d A or v dt d Q dt d P v [ ] [ ] [ ] [ ] = = = − = − A + B → P + Q [ ][ ] [ ] k A B dt d A v = − =