正在加载图片...
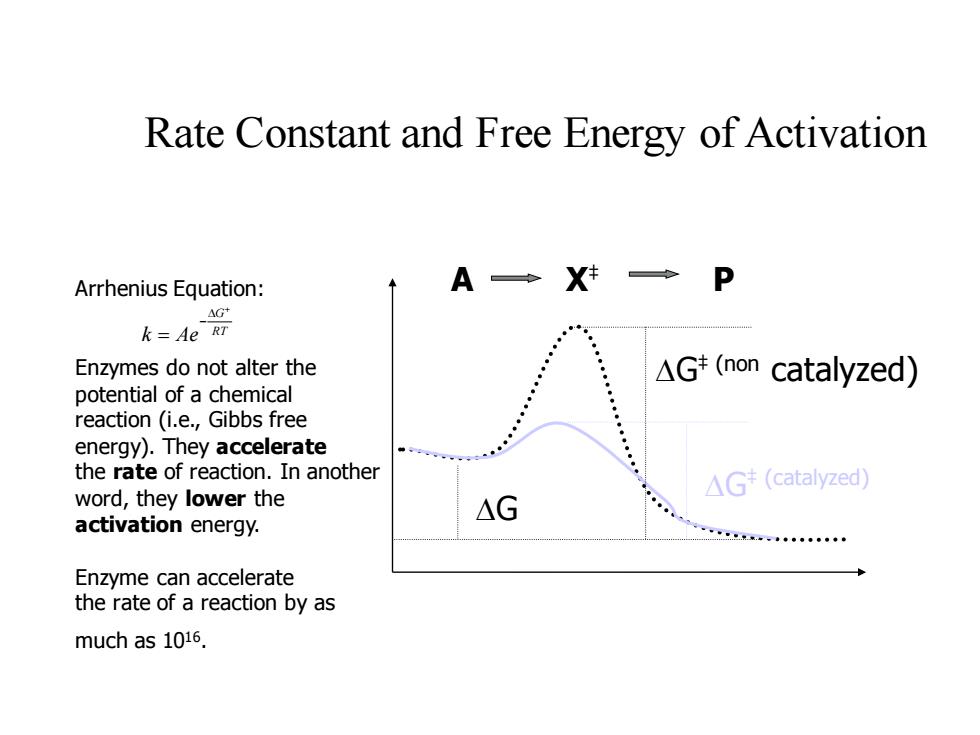
Rate Constant and Free Energy of Activation Arrhenius Equation: A◆X→P AG+ k=Ae RT Enzymes do not alter the △Gt(non catalyzed) potential of a chemical reaction (i.e.,Gibbs free energy).They accelerate the rate of reaction.In another △G(catalyzed word,they lower the △G activation energy. 二k。◆ Enzyme can accelerate the rate of a reaction by as much as 1016. Rate Constant and Free Energy of Activation DG‡ (non catalyzed) DG‡ (catalyzed) DG Arrhenius Equation: A X‡ P Enzymes do not alter the potential of a chemical reaction (i.e., Gibbs free energy). They accelerate the rate of reaction. In another word, they lower the activation energy. Enzyme can accelerate the rate of a reaction by as much as 1016 . RT G k Ae + D − =