正在加载图片...
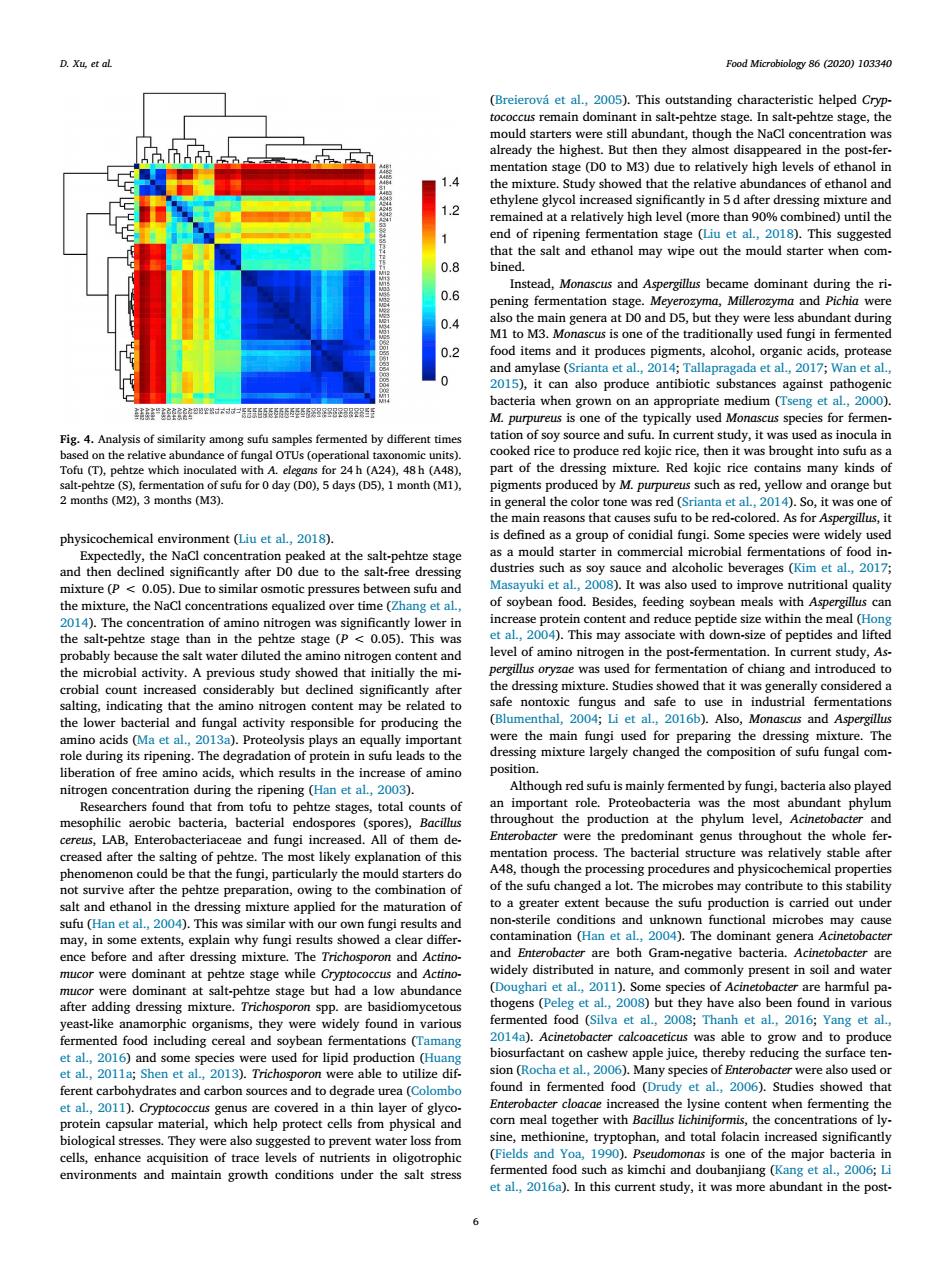
D.Xu et al Food Microbiology 86(2020)103340 (Breierov et al.2005).This outstanding charac the ed in the po the mixtur tha t the relative ab dances of ethanol an 12 0.8 制 M the ri. 0.6 fer ntation stage nd 0.4 ey wer M3.M sed fungi i 70ta201 ro lso pro against one of the typic ally used A uceed kojic rice, then it was brought into which in lo As fo phys ical environment (Liu et al,2018) tion of chi but cine the ingmixture largely changed the n of su ugh rec sufu is mainly ferr nted by to p ze stages,total of hou ction a LAB,Enteroba and fungi in ed.All of them d gre 004 efore and after dressing mixtu and and alt-peh tage but had a lo dely found ding cere d for 013.1 )help p of glyo ith They were d to prevent water loss fro maior environments and maintain growth conditions under the salt stressphysicochemical environment (Liu et al., 2018). Expectedly, the NaCl concentration peaked at the salt-pehtze stage and then declined significantly after D0 due to the salt-free dressing mixture (P < 0.05). Due to similar osmotic pressures between sufu and the mixture, the NaCl concentrations equalized over time (Zhang et al., 2014). The concentration of amino nitrogen was significantly lower in the salt-pehtze stage than in the pehtze stage (P < 0.05). This was probably because the salt water diluted the amino nitrogen content and the microbial activity. A previous study showed that initially the microbial count increased considerably but declined significantly after salting, indicating that the amino nitrogen content may be related to the lower bacterial and fungal activity responsible for producing the amino acids (Ma et al., 2013a). Proteolysis plays an equally important role during its ripening. The degradation of protein in sufu leads to the liberation of free amino acids, which results in the increase of amino nitrogen concentration during the ripening (Han et al., 2003). Researchers found that from tofu to pehtze stages, total counts of mesophilic aerobic bacteria, bacterial endospores (spores), Bacillus cereus, LAB, Enterobacteriaceae and fungi increased. All of them decreased after the salting of pehtze. The most likely explanation of this phenomenon could be that the fungi, particularly the mould starters do not survive after the pehtze preparation, owing to the combination of salt and ethanol in the dressing mixture applied for the maturation of sufu (Han et al., 2004). This was similar with our own fungi results and may, in some extents, explain why fungi results showed a clear difference before and after dressing mixture. The Trichosporon and Actinomucor were dominant at pehtze stage while Cryptococcus and Actinomucor were dominant at salt-pehtze stage but had a low abundance after adding dressing mixture. Trichosporon spp. are basidiomycetous yeast-like anamorphic organisms, they were widely found in various fermented food including cereal and soybean fermentations (Tamang et al., 2016) and some species were used for lipid production (Huang et al., 2011a; Shen et al., 2013). Trichosporon were able to utilize different carbohydrates and carbon sources and to degrade urea (Colombo et al., 2011). Cryptococcus genus are covered in a thin layer of glycoprotein capsular material, which help protect cells from physical and biological stresses. They were also suggested to prevent water loss from cells, enhance acquisition of trace levels of nutrients in oligotrophic environments and maintain growth conditions under the salt stress (Breierová et al., 2005). This outstanding characteristic helped Cryptococcus remain dominant in salt-pehtze stage. In salt-pehtze stage, the mould starters were still abundant, though the NaCl concentration was already the highest. But then they almost disappeared in the post-fermentation stage (D0 to M3) due to relatively high levels of ethanol in the mixture. Study showed that the relative abundances of ethanol and ethylene glycol increased significantly in 5 d after dressing mixture and remained at a relatively high level (more than 90% combined) until the end of ripening fermentation stage (Liu et al., 2018). This suggested that the salt and ethanol may wipe out the mould starter when combined. Instead, Monascus and Aspergillus became dominant during the ripening fermentation stage. Meyerozyma, Millerozyma and Pichia were also the main genera at D0 and D5, but they were less abundant during M1 to M3. Monascus is one of the traditionally used fungi in fermented food items and it produces pigments, alcohol, organic acids, protease and amylase (Srianta et al., 2014; Tallapragada et al., 2017; Wan et al., 2015), it can also produce antibiotic substances against pathogenic bacteria when grown on an appropriate medium (Tseng et al., 2000). M. purpureus is one of the typically used Monascus species for fermentation of soy source and sufu. In current study, it was used as inocula in cooked rice to produce red kojic rice, then it was brought into sufu as a part of the dressing mixture. Red kojic rice contains many kinds of pigments produced by M. purpureus such as red, yellow and orange but in general the color tone was red (Srianta et al., 2014). So, it was one of the main reasons that causes sufu to be red-colored. As for Aspergillus, it is defined as a group of conidial fungi. Some species were widely used as a mould starter in commercial microbial fermentations of food industries such as soy sauce and alcoholic beverages (Kim et al., 2017; Masayuki et al., 2008). It was also used to improve nutritional quality of soybean food. Besides, feeding soybean meals with Aspergillus can increase protein content and reduce peptide size within the meal (Hong et al., 2004). This may associate with down-size of peptides and lifted level of amino nitrogen in the post-fermentation. In current study, Aspergillus oryzae was used for fermentation of chiang and introduced to the dressing mixture. Studies showed that it was generally considered a safe nontoxic fungus and safe to use in industrial fermentations (Blumenthal, 2004; Li et al., 2016b). Also, Monascus and Aspergillus were the main fungi used for preparing the dressing mixture. The dressing mixture largely changed the composition of sufu fungal composition. Although red sufu is mainly fermented by fungi, bacteria also played an important role. Proteobacteria was the most abundant phylum throughout the production at the phylum level, Acinetobacter and Enterobacter were the predominant genus throughout the whole fermentation process. The bacterial structure was relatively stable after A48, though the processing procedures and physicochemical properties of the sufu changed a lot. The microbes may contribute to this stability to a greater extent because the sufu production is carried out under non-sterile conditions and unknown functional microbes may cause contamination (Han et al., 2004). The dominant genera Acinetobacter and Enterobacter are both Gram-negative bacteria. Acinetobacter are widely distributed in nature, and commonly present in soil and water (Doughari et al., 2011). Some species of Acinetobacter are harmful pathogens (Peleg et al., 2008) but they have also been found in various fermented food (Silva et al., 2008; Thanh et al., 2016; Yang et al., 2014a). Acinetobacter calcoaceticus was able to grow and to produce biosurfactant on cashew apple juice, thereby reducing the surface tension (Rocha et al., 2006). Many species of Enterobacter were also used or found in fermented food (Drudy et al., 2006). Studies showed that Enterobacter cloacae increased the lysine content when fermenting the corn meal together with Bacillus lichiniformis, the concentrations of lysine, methionine, tryptophan, and total folacin increased significantly (Fields and Yoa, 1990). Pseudomonas is one of the major bacteria in fermented food such as kimchi and doubanjiang (Kang et al., 2006; Li et al., 2016a). In this current study, it was more abundant in the postFig. 4. Analysis of similarity among sufu samples fermented by different times based on the relative abundance of fungal OTUs (operational taxonomic units). Tofu (T), pehtze which inoculated with A. elegans for 24 h (A24), 48 h (A48), salt-pehtze (S), fermentation of sufu for 0 day (D0), 5 days (D5), 1 month (M1), 2 months (M2), 3 months (M3). D. Xu, et al. Food Microbiology 86 (2020) 103340 6