正在加载图片...
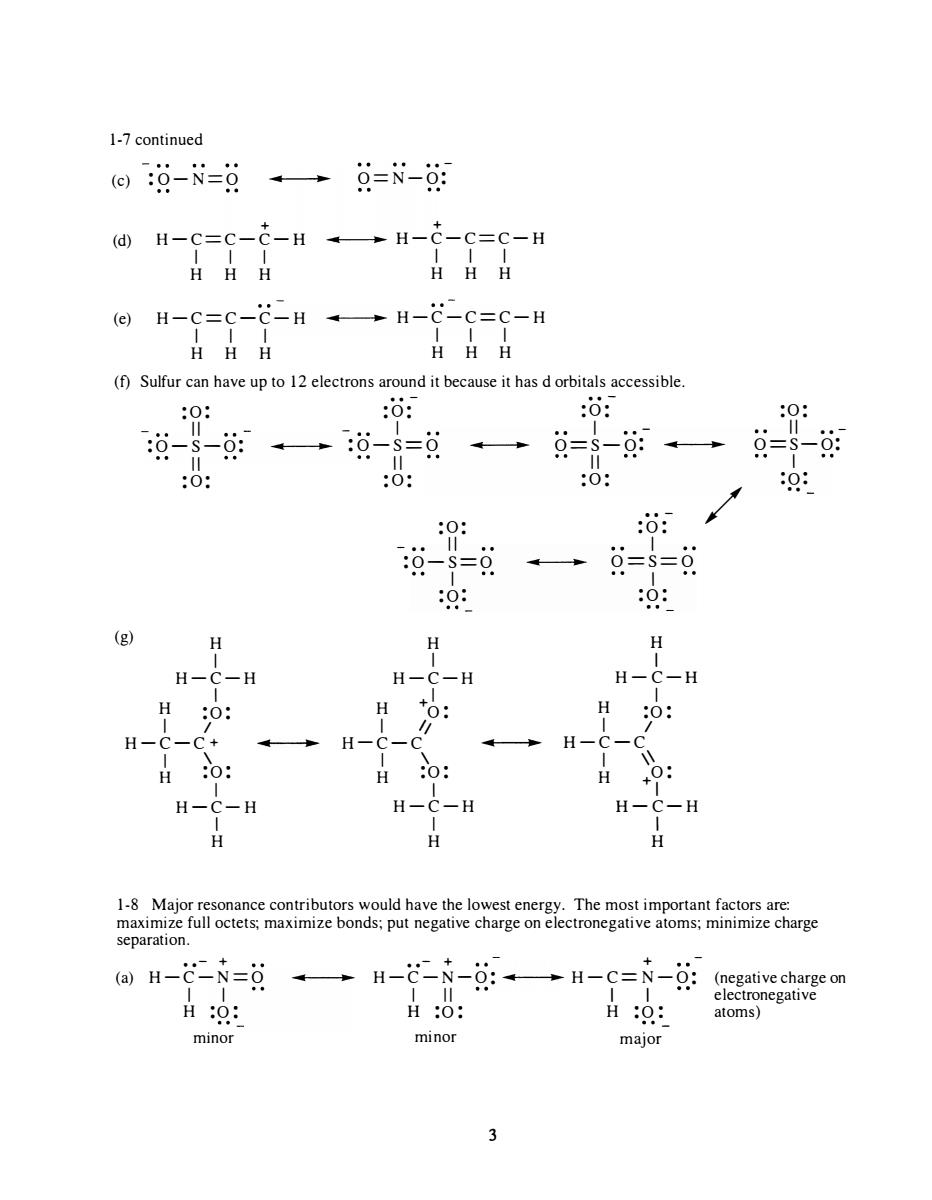
1-7continued 回:0-N=g→9=N-湖 H-c=c-c-H --H-c-c=c-H HHH HHH (f)Sulfur can have upto 12 electrons around it because it has dorbitals accessible. 0: :0: :o: 0: :0: 0 0: 0 9-=9→==g 0: 0: (g) H H H -C-H H -C-H H-C-H H H H 0 H H H 0 H-C-H H-C-H H-C-H H separation. ()H-C-N=8 →H-N-g:→H-C=N-女:((negative H0: H:O: H:0: atoms)gativ minor minor major 3 1-7 continued .. - (c) :O-N=O .. .. O=N-O: + + (d) H-C=C-C-H .. .. H-C-C=C-H I I I I I I H H H H H H (e) H-C=C-C- H .. .. H-C-C=C-H I I I I I I H H H H H H (f) Sulfur can have up to 12 electrons around it because it has d orbitals accessible . (g) : 0: II :O- S- O: II : 0: H I H-C-H I H :0 : I I H-C- C+ I \ H :0 : I H-C-H I H .. .. - : 0: I .. .. :O -S=O .. .. II : 0: H I :0: - •• II :O- S=O I :0: H I H-C-H I + 0: 1/ H- C-C .. I \ H :0 : I H-C-H I H .. . . - :0 : I O==S-O: .. .. II :0 : . . - :0: I .. .. O==S==O I :0 : H I H-C-H I H :0 : I I .. H-C-C I \\ H + 0: I H- C-H I H / :0 : II •• - O=S-O: I :0 : . . 1-8 Major resonance contributors would have the lowest energy. The most important factors are: maximize full octets; maximize bonds; put negative charge on electronegative atoms; minimize charge separation. • • - + . .- + + (a) H-C-N=O .. .. H-C-N-O: .. .. H-C=N- O: (negative charge on I I I II I I electronegative H :0 : H :0 : H :0 : atoms) mInor minor major 3