正在加载图片...
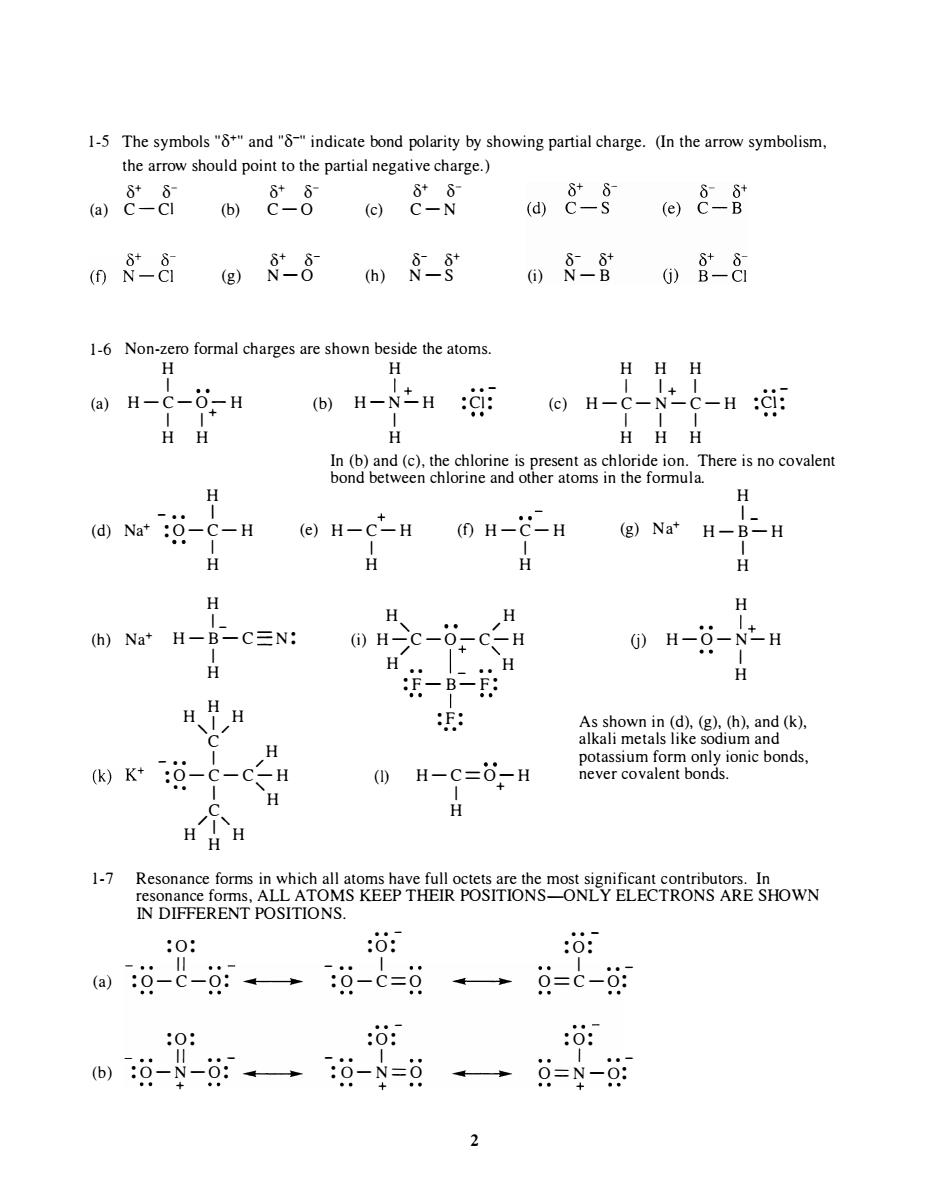
1-5 The symbols"and"indicate bond polarity by showing partial charge.(In the arrow symbolism the arrow should point to the partial negative charge.) 088088 08- 间&§@8-8 0-8g8-8m§-8 0§-含088 1-6 Non-zero formal charges are shown beside the atoms H-N之H: HHH In (b)and (c)the chlorine as chloride ion.There is no covalen bond betwe choeomim he omula H C-H (e)H-C*-H (1)H-C-H (g)Na'H-B-H H H H h)Na*H-B-C三N: H-C-6CH H-O-N-H H H、HH (k)K*-c-c ()H-C=0-H HH H Reeo6N5代6来"零TRONS ARE SHOWN 1-7 Resonance forms in which all aton IN DIFFERENT POSITIONS. :0: :o: :o: a识-c-g:→过-0=9·→总=C-09 0: :o: 过--0。过-8=日一总=9-每 21-5 The symbols "8+" and "8-" indicate bond polarity by showing partial charge. (In the arrow symbolism, the arrow should point to the partial negative charge.) � � � � � � (a) C-C1 (b) C - O (c) C-N 1-6 (a) 8+ 8- 8- 8+ (g) N-O (h) N -S Non-zero formal charges are shown beside the atoms. H H 1 1 + .. - H-C-O-H (b) H-N-H : C1: 1 1 + 1 H H H 8- 8+ (i) N-B H 1 H H 1+ 1 (c) H-C-N-C-H 1 1 1 H H H .. - :Cl: In (b) and (c), the chlorine is present as chloride ion. There is no covalent bond between chlorine and other atoms in the formula. H - • • I + H 1- (d) Na+ :O - C - H (e) H-C-H (f) H - C-H 1 (g) Na+ H - B-H (h) Na+ I H H 1 - H-B-C- N: I H H H H ,1/ C H - •• 1 / ·• O- C-C-H • • I ' C H /1' H H H 1 H H H , / H (i) H-C-O-C-H / 1 + H •• _ •• ' H (I) :F-B-F: 1 : F: .. H-C= O- H 1 + H U) I H H 1 H - O-N-H + I H As shown in (d), (g), (h), and (k), alkali metals like sodium and potassium form only ionic bonds, never covalent bonds. 1-7 Resonance forms in which all atoms have full octets are the most significant contributors. In resonance forms, ALL ATOMS KEEP THEIR POSITIONS-ONLY ELECTRONS ARE SHOWN IN DIFFERENT POSITIONS. :0 : " (a) :O- C-O: .. .. :0 : " . . - (b) :O -N-O: .. .. + . . - : 0: - .. 1 :O- C=O . . - :0 : - .. 1 :O- N=O + .. ... 2 .. - : 0: 1 •• - O= C-O: : 0: I •• - O=N - O: +