正在加载图片...
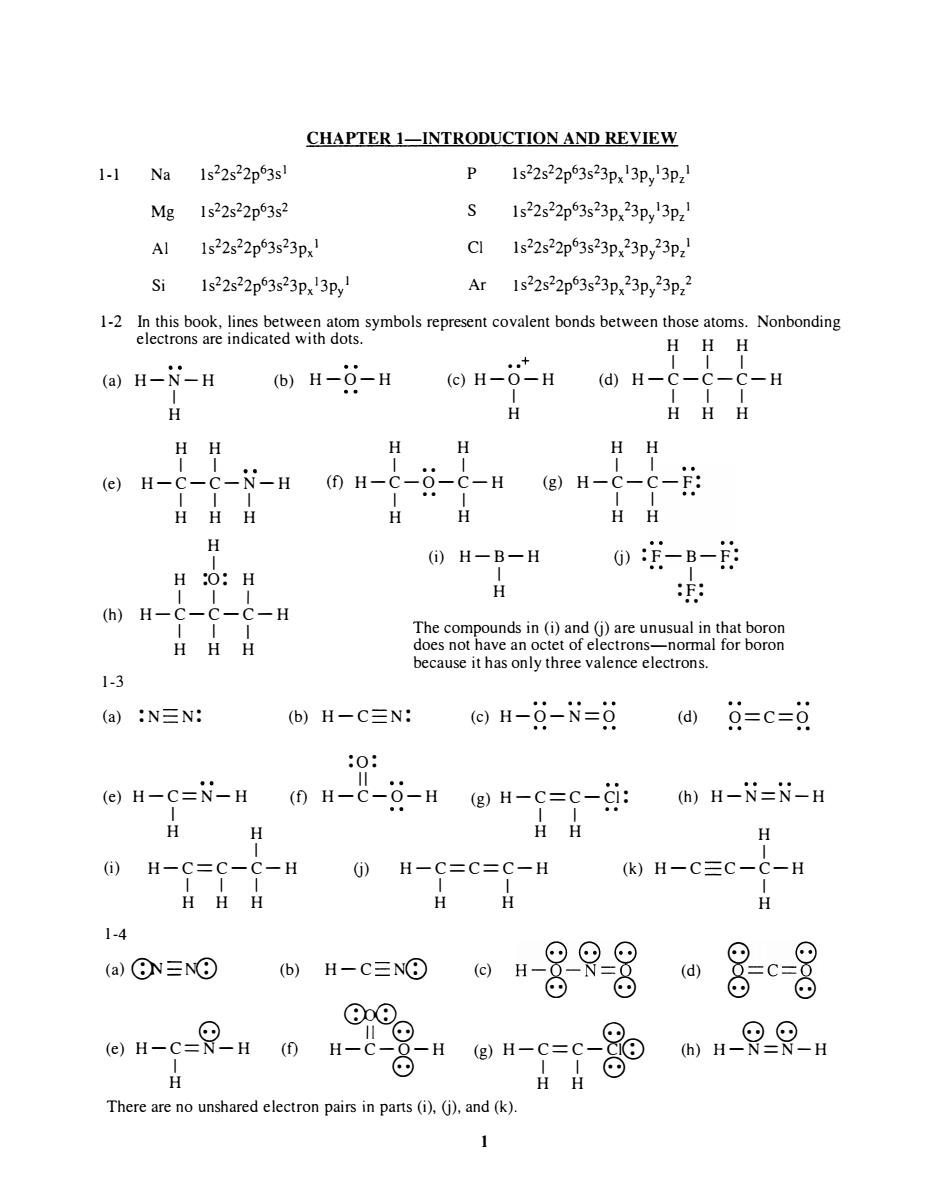
CHAPTER 1-INTRODUCTION AND REVIEW 1-1Na1s22s22p3s P 1s22s22p63s23px3p3pz Mg 1s22s22p63s2 Al 1s22s22p63s23px1 Cl Is22s22p63s23p,23p,23p2 Si 1s22s22p3s23p3p Ar1s22s22p3s23p,23p,23p2 HHH (a)H-N-H (b)H-6-H ⊙H-0-H@H- -H HH HH eH-C---HmH-C--C-HH-C-- HHH H HH H (i)H-B-H 0-B-的 H :O:H H (h)H-C-C-C-H The c HHH vreroorom because it has only three valence electrons. 1-3 (a):N三N: b)H-C三N @)H-9-N=9@)9=c=g eH-C=N-H0H-C-Q-HgH-c=c-年:)H-i=N-H H H 1-4 (a)⑧N三N⊙ 仙-0日8是8 ⊙⊙ on-e-Rn 0 n-8u n-e-g H There are no unshared electron pairs in pars(i).(j).and(k). 1-1 Na Mg AI Si CHAPTER I-INTRODUCTION AND REVIEW 1 s22s22p 6 3s 1 1 s 22s 22p 6 3s2 Is 22s 22p 6 3s 23px 1 1 s22s 22p 6 3s 2 3px 13p y I P S CI Ar 1 s22s 22p 6 3s 2 3px 13p y 13pz 1 1 s22s 22p 6 3s 2 3px 23p y 13pz 1 1 s22s 22p 6 3s 2 3px 23p y 23pz 1 1 s 2 2s22p 6 3s 2 3px 23p y 23pz 2 1-2 In this book, lines between atom symbols represent covalent bonds between those atoms. Nonbonding electrons are indicated with dots. H H H •• + I I I (a) H -N-H I (b) H - O-H (c) H-O-H (d) H-C- C-C-H H H H I I (e) H-C-C- N -H I I I H H H H I H :0: H I I I (h) H-C-C-C-H 1-3 I I I H H H H H I • • I (f) H -C-O-C-H I • • I H H I I I I H H H H H H I I (g) H-C - C - F: I I H H (i) H -B - H I (j) : F-B-F: - - I H : F: .. The compounds in (i) and (j) are unusual in that boron does not have an octet of electrons-normal for boron because it has only three valence electrons. (a) :N N: (b) H - C N: (c) H-O - N=O (d) O==C=O (e) H -C=N- H I H H :0 : II (f) H-C-O-H (g) H -C == C - CI : I I •• H H (h) H -N=N-H H I (i) I H-C==C-C-H (j) H - C==C==C-H (k) H-C C - C-H 1-4 I I I H H H (a) G}J -NQ) 8 (e) H - C=N - H I H (b) (f) H-C NeD C)J(D I I H H (c) 88(.:) H - O-N O 0) 0) • II ·8 H-C-O-H 8 8 (g) H - C == C - CleD I I 8 H H There are no unshared electron pairs in parts (i), (j), and (k). 1 (d) I H 0) 0) o == c=o 0) 0) 88 (h) H-N=N-H