正在加载图片...
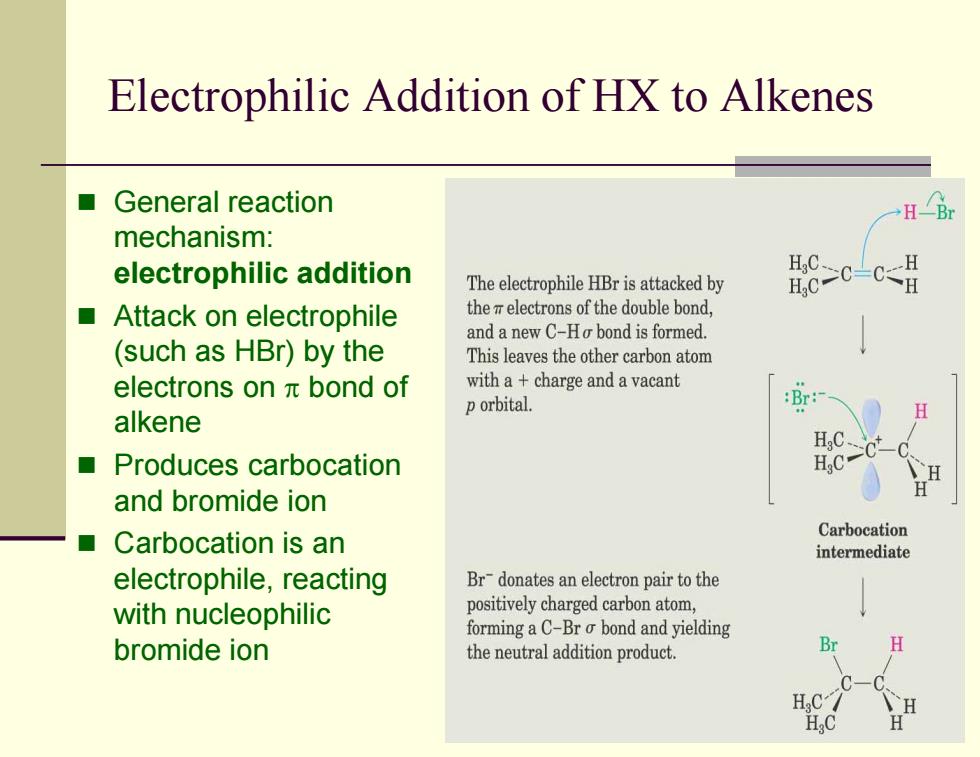
Electrophilic Addition of HX to Alkenes General reaction H∠Br mechanism: electrophilic addition The electrophile HBr is attacked by Attack on electrophile the electrons of the double bond, and a new C-Ho bond is formed. (such as HBr))by the This leaves the other carbon atom electrons onπbond of with a+charge and a vacant p orbital. alkene H HC、 Produces carbocation H.C" and bromide ion Carbocation is an Carbocation ■ intermediate electrophile,reacting Br-donates an electron pair to the with nucleophilic positively charged carbon atom, forming a C-Br o bond and yielding bromide ion the neutral addition product. H H.C ,C Electrophilic Addition of HX to Alkenes General reaction mechanism: electrophilic addition Attack on electrophile (such as HBr) by the electrons on π bond of alkene Produces carbocation and bromide ion Carbocation is an electrophile, reacting with nucleophilic bromide ion