正在加载图片...
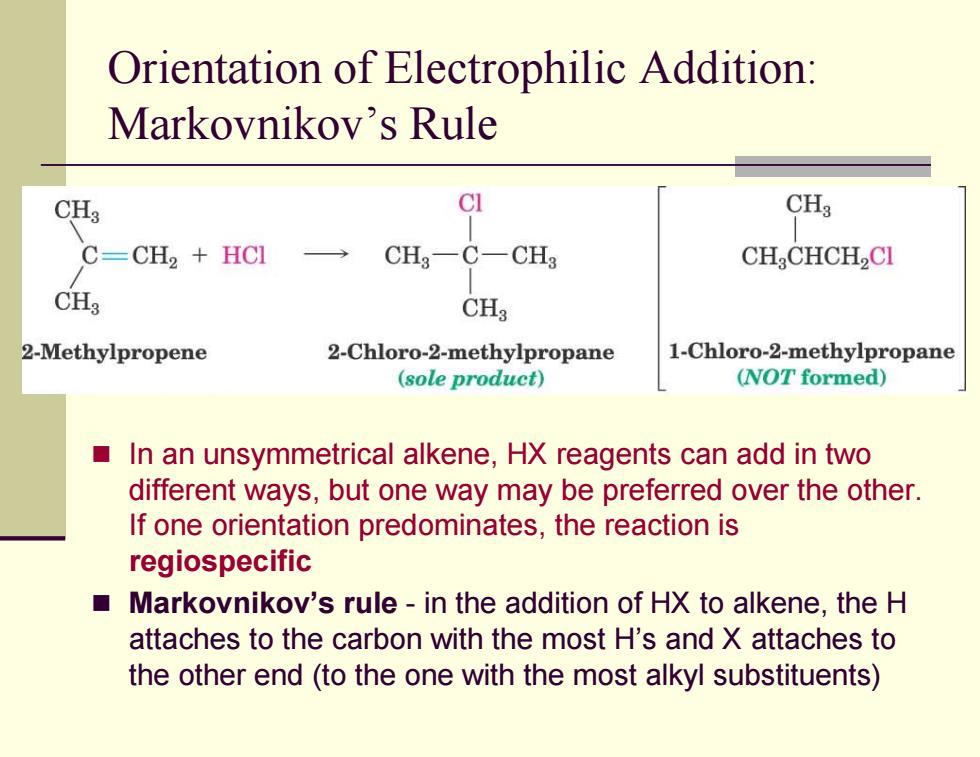
Orientation of Electrophilic Addition: Markovnikov's Rule CH3 CI CH3 C=CH2 +HCI CH3一C-CH3 CHCHCH,CI CH3 CHg 2-Methylpropene 2-Chloro-2-methylpropane 1-Chloro-2-methylpropane (sole product) (NOT formed) In an unsymmetrical alkene,HX reagents can add in two different ways,but one way may be preferred over the other. If one orientation predominates,the reaction is regiospecific Markovnikov's rule-in the addition of HX to alkene,the H attaches to the carbon with the most H's and X attaches to the other end(to the one with the most alkyl substituents)Orientation of Electrophilic Addition: Markovnikov’s Rule In an unsymmetrical alkene, HX reagents can add in two different ways, but one way may be preferred over the other. If one orientation predominates, the reaction is regiospecific Markovnikov’s rule - in the addition of HX to alkene, the H attaches to the carbon with the most H’s and X attaches to the other end (to the one with the most alkyl substituents)