正在加载图片...
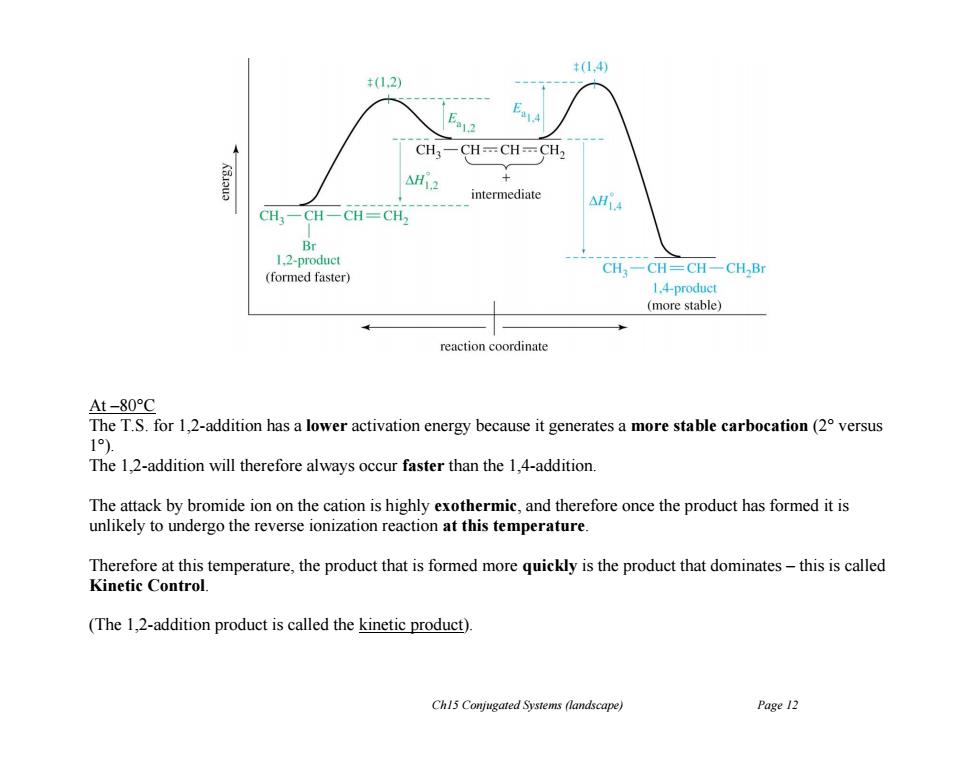
t(1.4) +(1.2) CH3-CH-CH=CH2 AH12 + intermediate △HiA CH一CH-CH=CH 1.2-product CH3一CH=CH一CH,Br (formed faster) 1.4-product (more stable) reaction coordinate At-80°C The T.S.for 1,2-addition has a lower activation energy because it generates a more stable carbocation(2 versus 1). The 1,2-addition will therefore always occur faster than the 1,4-addition. The attack by bromide ion on the cation is highly exothermic,and therefore once the product has formed it is unlikely to undergo the reverse ionization reaction at this temperature. Therefore at this temperature,the product that is formed more quickly is the product that dominates-this is called Kinetic Control. (The 1,2-addition product is called the kinetic product). Ch15 Conjugated Systems (landscape) Page 12Ch15 Conjugated Systems (landscape) Page 12 At –80°C The T.S. for 1,2-addition has a lower activation energy because it generates a more stable carbocation (2° versus 1°). The 1,2-addition will therefore always occur faster than the 1,4-addition. The attack by bromide ion on the cation is highly exothermic, and therefore once the product has formed it is unlikely to undergo the reverse ionization reaction at this temperature. Therefore at this temperature, the product that is formed more quickly is the product that dominates – this is called Kinetic Control. (The 1,2-addition product is called the kinetic product)