正在加载图片...
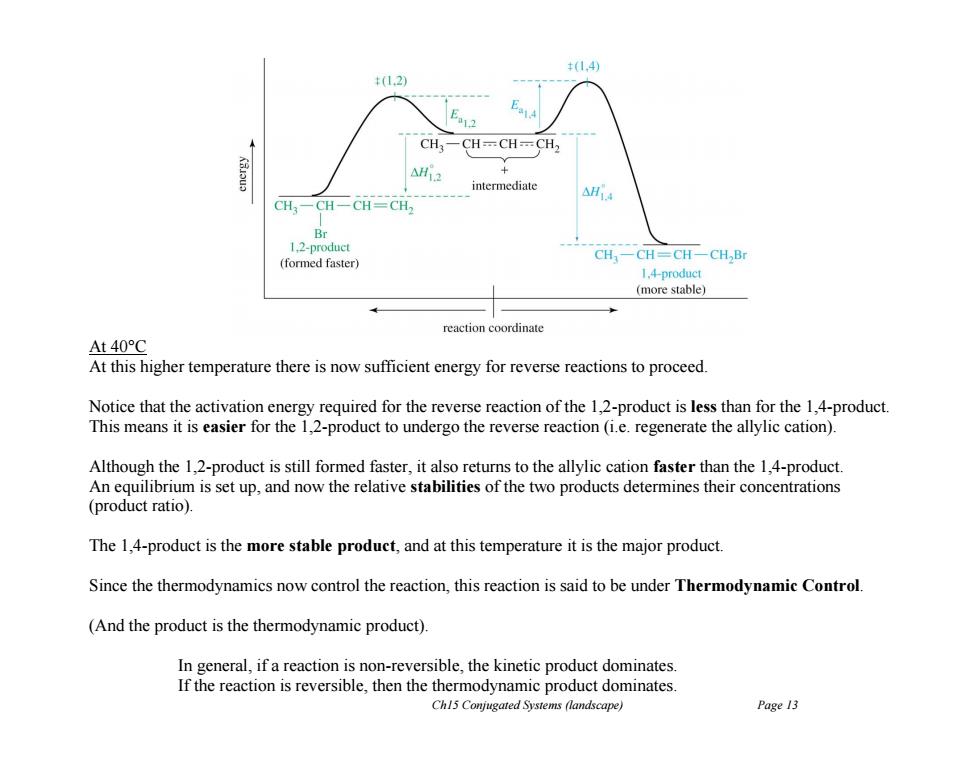
(1.4) 12) E2 CH3一CH=CH=CH2 intermediate CH3- Br 1.2-product (formed faster) CH-CH=CH-CH2Br 1.4-product (more stable) reaction coordinate At40°C At this higher temperature there is now sufficient energy for reverse reactions to proceed Notice that the activation energy required for the reverse reaction of the 1,2-product is less than for the 1,4-product This means it is easier for the 1,2-product to undergo the reverse reaction(ie.regenerate the allylic cation). Although the 1,2-product is still formed faster,it also returns to the allylic cation faster than the 1,4-product. An equilibrium is set up,and now the relative stabilities of the two products determines their concentrations (product ratio). The 1,4-product is the more stable product,and at this temperature it is the major product. Since the thermodynamics now control the reaction,this reaction is said to be under Thermodynamic Control. (And the product is the thermodynamic product). In general,if a reaction is non-reversible,the kinetic product dominates If the reaction is reversible,then the thermodynamic product dominates Ch15 Conjugated Systems (landscape) Page 13 Ch15 Conjugated Systems (landscape) Page 13 At 40°C At this higher temperature there is now sufficient energy for reverse reactions to proceed. Notice that the activation energy required for the reverse reaction of the 1,2-product is less than for the 1,4-product. This means it is easier for the 1,2-product to undergo the reverse reaction (i.e. regenerate the allylic cation). Although the 1,2-product is still formed faster, it also returns to the allylic cation faster than the 1,4-product. An equilibrium is set up, and now the relative stabilities of the two products determines their concentrations (product ratio). The 1,4-product is the more stable product, and at this temperature it is the major product. Since the thermodynamics now control the reaction, this reaction is said to be under Thermodynamic Control. (And the product is the thermodynamic product). In general, if a reaction is non-reversible, the kinetic product dominates. If the reaction is reversible, then the thermodynamic product dominates