正在加载图片...
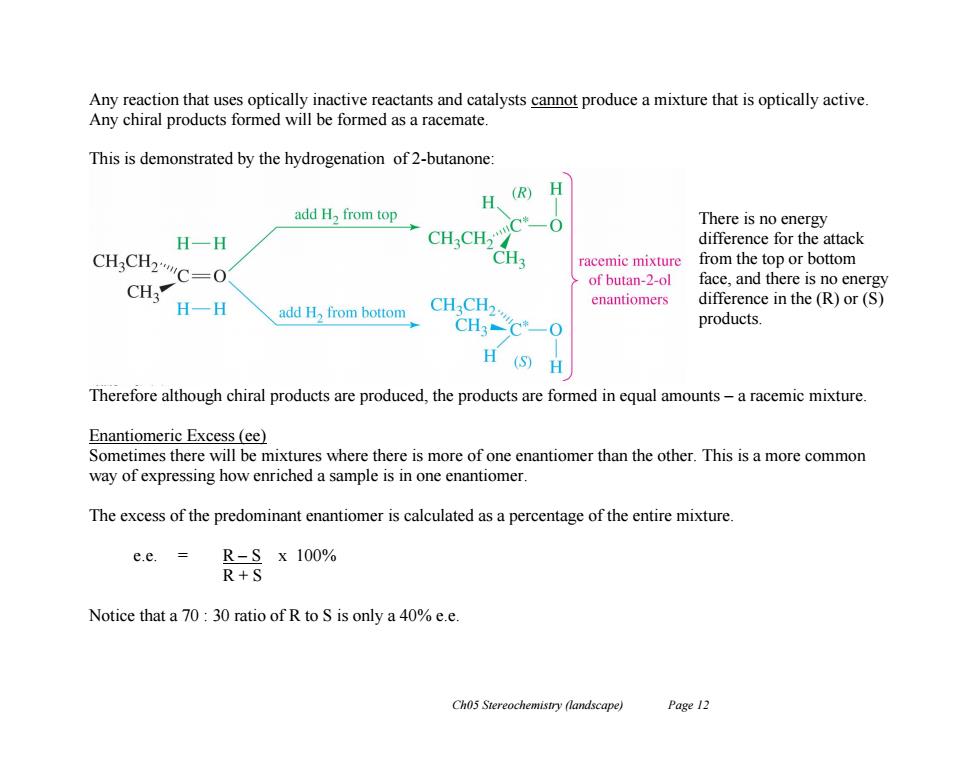
Any reaction that uses optically inactive reactants and catalysts cannot produce a mixture that is optically active. Any chiral products formed will be formed as a racemate. This is demonstrated by the hydrogenation of 2-butanone: H (R) H add H,from top There is no energy H一H CH CH2 difference for the attack CHaCH2C=O CH3 racemic mixture from the top or bottom of butan-2-ol face,and there is no energy CH3 H-H CHCH enantiomers difference in the(R)or(S) add H,from bottom CH3-C*-0 products. H (S)H Therefore although chiral products are produced,the products are formed in equal amounts-a racemic mixture. Enantiomeric Excess (ee) Sometimes there will be mixtures where there is more of one enantiomer than the other.This is a more common way of expressing how enriched a sample is in one enantiomer. The excess of the predominant enantiomer is calculated as a percentage of the entire mixture. e.e.= R-Sx100% R+S Notice that a 70:30 ratio of R to S is only a 40%e.e Ch05 Stereochemistry (landscape) Page 12Ch05 Stereochemistry (landscape) Page 12 Any reaction that uses optically inactive reactants and catalysts cannot produce a mixture that is optically active. Any chiral products formed will be formed as a racemate. This is demonstrated by the hydrogenation of 2-butanone: There is no energy difference for the attack from the top or bottom face, and there is no energy difference in the (R) or (S) products. Therefore although chiral products are produced, the products are formed in equal amounts – a racemic mixture. Enantiomeric Excess (ee) Sometimes there will be mixtures where there is more of one enantiomer than the other. This is a more common way of expressing how enriched a sample is in one enantiomer. The excess of the predominant enantiomer is calculated as a percentage of the entire mixture. e.e. = R – S x 100% R + S Notice that a 70 : 30 ratio of R to S is only a 40% e.e