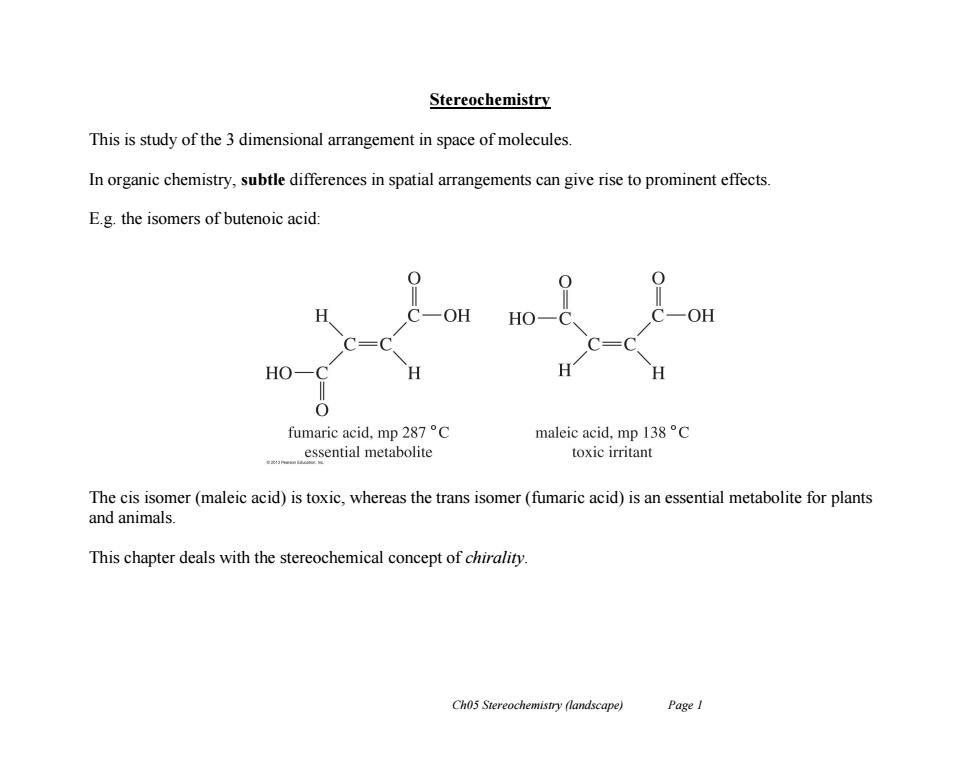
Stereochemistry This is study of the 3 dimensional arrangement in space of molecules In organic chemistry,subtle differences in spatial arrangements can give rise to prominent effects. E.g.the isomers of butenoic acid: H OH HO -oh HO fumaric acid,mp287°C maleic acid,mpl38°C essential metabolite toxic irritant The cis isomer(maleic acid)is toxic,whereas the trans isomer(fumaric acid)is an essential metabolite for plants and animals This chapter deals with the stereochemical concept of chirality. Ch05 Stereochemistry (landscape) PageI
Ch05 Stereochemistry (landscape) Page 1 Stereochemistry This is study of the 3 dimensional arrangement in space of molecules. In organic chemistry, subtle differences in spatial arrangements can give rise to prominent effects. E.g. the isomers of butenoic acid: The cis isomer (maleic acid) is toxic, whereas the trans isomer (fumaric acid) is an essential metabolite for plants and animals. This chapter deals with the stereochemical concept of chirality
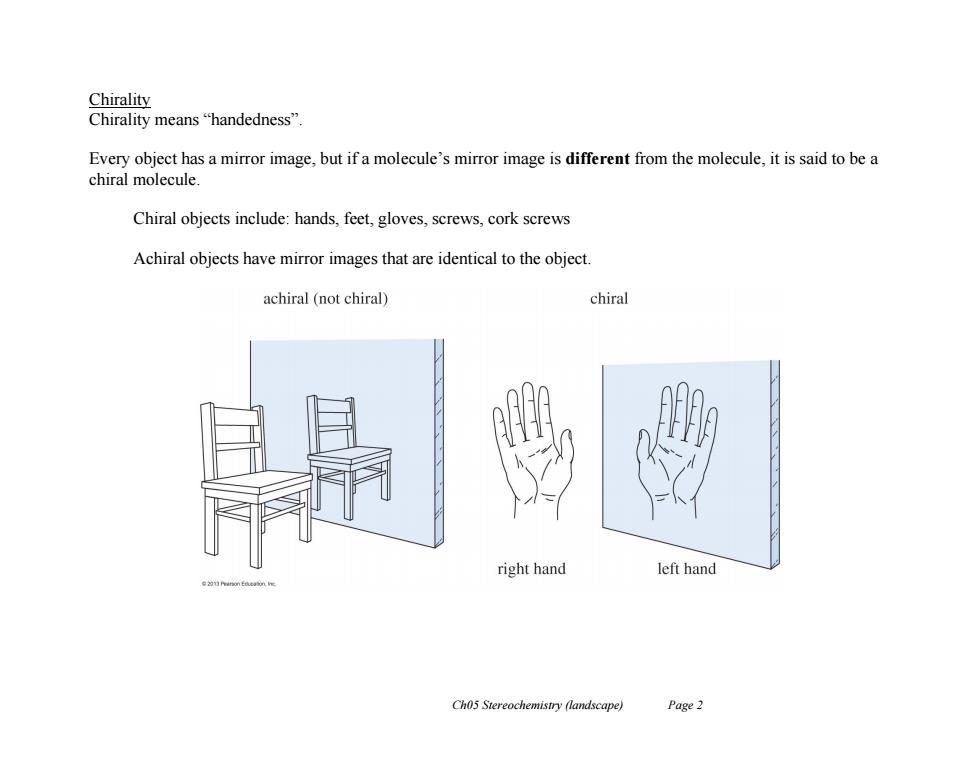
Chirality Chirality means"handedness". Every object has a mirror image,but if a molecule's mirror image is different from the molecule,it is said to be a chiral molecule. Chiral objects include:hands,feet,gloves,screws,cork screws Achiral objects have mirror images that are identical to the object. achiral(not chiral) chiral right hand left hand Cho5 Stereochemistry (landscape) Page 2
Ch05 Stereochemistry (landscape) Page 2 Chirality Chirality means “handedness”. Every object has a mirror image, but if a molecule’s mirror image is different from the molecule, it is said to be a chiral molecule. Chiral objects include: hands, feet, gloves, screws, cork screws Achiral objects have mirror images that are identical to the object
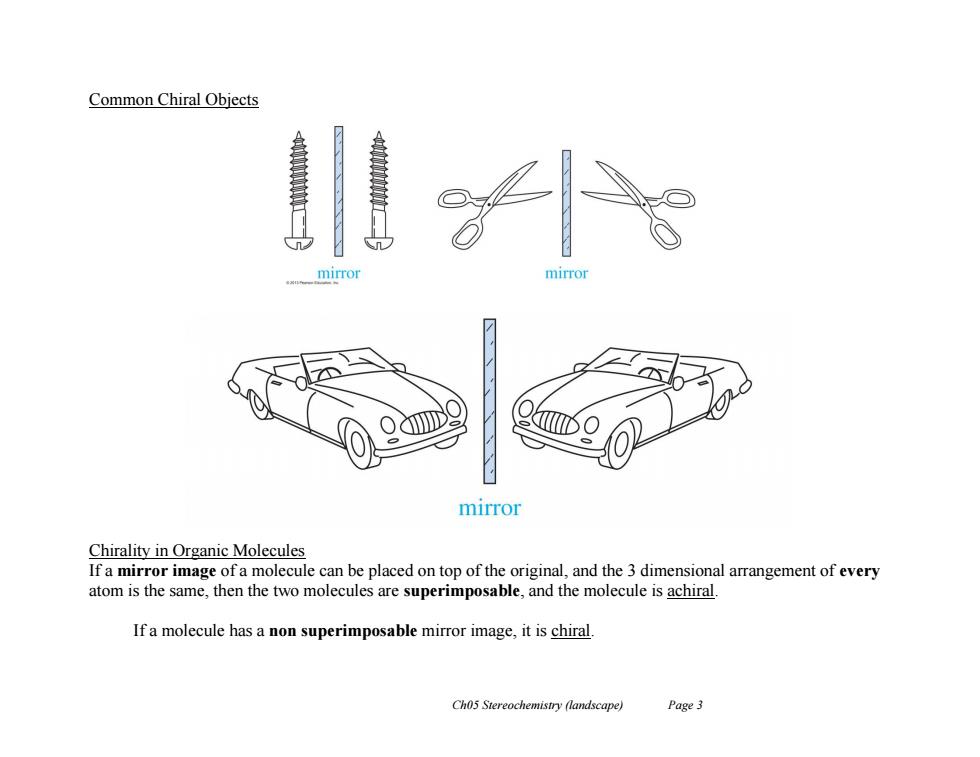
Common Chiral Objects mirror mirror mirror Chirality in Organic Molecules If a mirror image of a molecule can be placed on top of the original,and the 3 dimensional arrangement of every atom is the same,then the two molecules are superimposable,and the molecule is achiral. If a molecule has a non superimposable mirror image,it is chiral. Ch05 Stereochemistry (landscape) Page 3
Ch05 Stereochemistry (landscape) Page 3 Common Chiral Objects Chirality in Organic Molecules If a mirror image of a molecule can be placed on top of the original, and the 3 dimensional arrangement of every atom is the same, then the two molecules are superimposable, and the molecule is achiral. If a molecule has a non superimposable mirror image, it is chiral
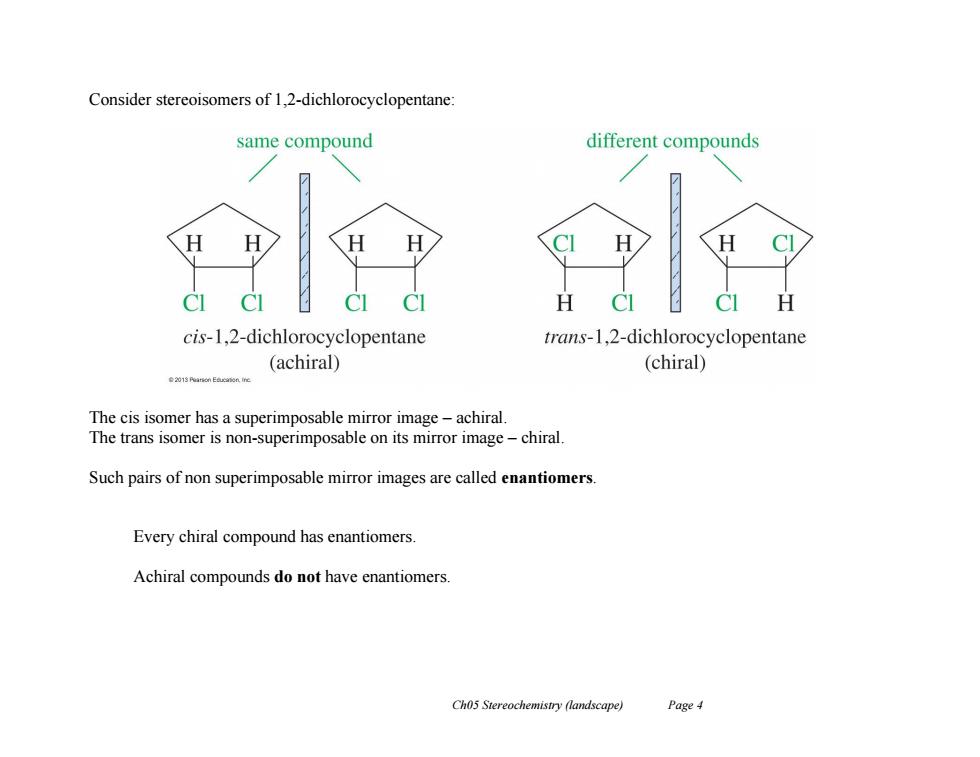
Consider stereoisomers of 1,2-dichlorocyclopentane: same compound different compounds H H cis-1,2-dichlorocyclopentane trans-1,2-dichlorocyclopentane (achiral) (chiral) ●201白onEonir The cis isomer has a superimposable mirror image-achiral. The trans isomer is non-superimposable on its mirror image-chiral. Such pairs of non superimposable mirror images are called enantiomers. Every chiral compound has enantiomers. Achiral compounds do not have enantiomers. Ch05 Stereochemistry (landscape) Page 4
Ch05 Stereochemistry (landscape) Page 4 Consider stereoisomers of 1,2-dichlorocyclopentane: The cis isomer has a superimposable mirror image – achiral. The trans isomer is non-superimposable on its mirror image – chiral. Such pairs of non superimposable mirror images are called enantiomers. Every chiral compound has enantiomers. Achiral compounds do not have enantiomers
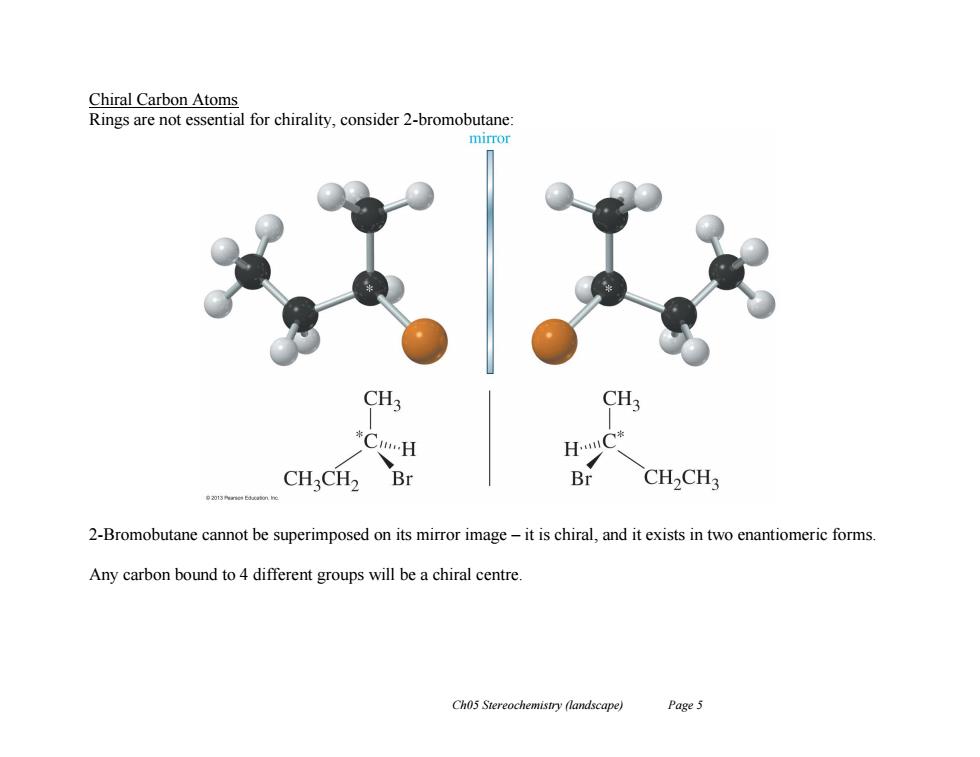
Chiral Carbon Atoms Rings are not essential for chirality,consider 2-bromobutane: mirror CH; CH; *C…H H.C* CH2CH2 Br Br CH>CH3 2-Bromobutane cannot be superimposed on its mirror image-it is chiral,and it exists in two enantiomeric forms. Any carbon bound to 4 different groups will be a chiral centre Ch05 Stereochemistry (landscape) Page5
Ch05 Stereochemistry (landscape) Page 5 Chiral Carbon Atoms Rings are not essential for chirality, consider 2-bromobutane: 2-Bromobutane cannot be superimposed on its mirror image – it is chiral, and it exists in two enantiomeric forms. Any carbon bound to 4 different groups will be a chiral centre
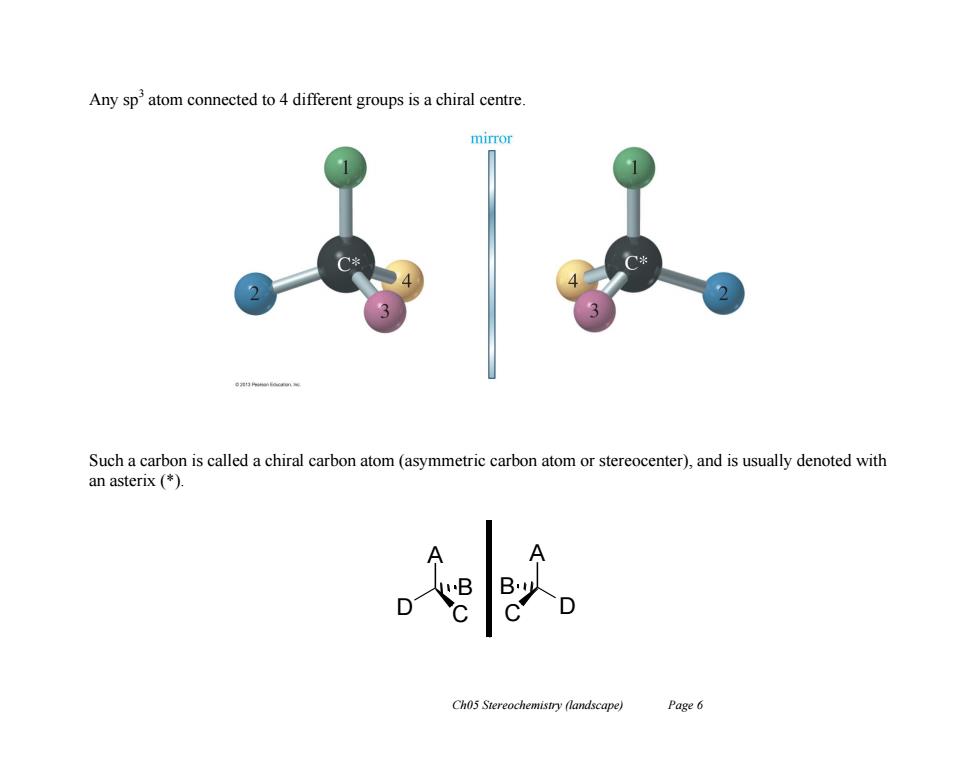
Any sp2 atom connected to 4 different groups is a chiral centre. mirror Such a carbon is called a chiral carbon atom(asymmetric carbon atom or stereocenter),and is usually denoted with an asterix (*) Ch05 Stereochemistry (landscape) Page 6
Ch05 Stereochemistry (landscape) Page 6 Any sp3 atom connected to 4 different groups is a chiral centre. Such a carbon is called a chiral carbon atom (asymmetric carbon atom or stereocenter), and is usually denoted with an asterix (*). A C D B A D C B

(R)and (S)Assignments To distinguish between the two enantiomers,one is called the(S)enantiomer,and the other the(R)enantiomer The(R)and(S)assignments are designated via the Cahn-Ingold-Prelog Convention. Each chiral center is designated either(R)or(S) There are two steps to assigning(R)or(S)to an enantiomer. 1)Assignment of"priority"to the groups bound to the asymmetric carbon 2)Using the“priority'”to decide on(R)or(S). 1)Assignment of Priority We look at the atoms directly bound to chiral carbon (a)Atoms with higher atomic numbers receive higher priorities. I>S>0>N>3℃>12C>Li>3H(T>2H(D)>H (b)In the case of the same atoms being bound directly to the chiral carbon,we go to the next atoms along the chain. -CH2Br>-CHCl2>-C(CH3)3>-CH(CH3)CH2F>-CH(CH3)2>-CH2CH; (c)Double and triple bonds are treated as if each bond were to a separate atom.(Imagine that each it bond is broken and that the atoms at both ends were duplicated). Ch05 Stereochemistry (landscape) Page 7
Ch05 Stereochemistry (landscape) Page 7 (R) and (S) Assignments To distinguish between the two enantiomers, one is called the (S) enantiomer, and the other the (R) enantiomer. The (R) and (S) assignments are designated via the Cahn-Ingold-Prelog Convention. Each chiral center is designated either (R) or (S). There are two steps to assigning (R) or (S) to an enantiomer: 1) Assignment of “priority” to the groups bound to the asymmetric carbon 2) Using the “priority” to decide on (R) or (S). 1) Assignment of Priority We look at the atoms directly bound to chiral carbon. (a) Atoms with higher atomic numbers receive higher priorities. I > S > O > N > 13C > 12C > Li > 3H (T) > 2H (D) > H (b) In the case of the same atoms being bound directly to the chiral carbon, we go to the next atoms along the chain. -CH2Br > -CHCl2 > -C(CH3)3 > -CH(CH3)CH2F > -CH(CH3)2 > -CH2CH3 (c) Double and triple bonds are treated as if each bond were to a separate atom. (Imagine that each bond is broken and that the atoms at both ends were duplicated)
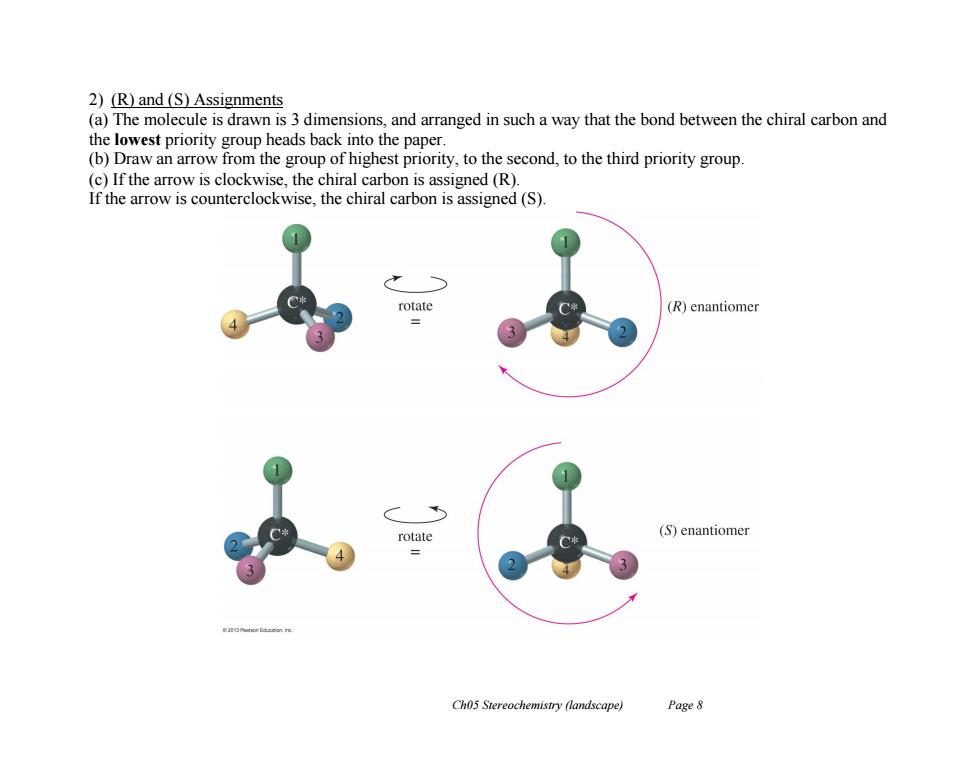
2)(R)and(S)Assignments (a)The molecule is drawn is 3 dimensions,and arranged in such a way that the bond between the chiral carbon and the lowest priority group heads back into the paper. (b)Draw an arrow from the group of highest priority,to the second,to the third priority group. (c)If the arrow is clockwise,the chiral carbon is assigned (R). If the arrow is counterclockwise,the chiral carbon is assigned (S) rotate (R)enantiomer rotate (S)enantiomer = Ch05 Stereochemistry (landscape) Page 8
Ch05 Stereochemistry (landscape) Page 8 2) (R) and (S) Assignments (a) The molecule is drawn is 3 dimensions, and arranged in such a way that the bond between the chiral carbon and the lowest priority group heads back into the paper. (b) Draw an arrow from the group of highest priority, to the second, to the third priority group. (c) If the arrow is clockwise, the chiral carbon is assigned (R). If the arrow is counterclockwise, the chiral carbon is assigned (S)
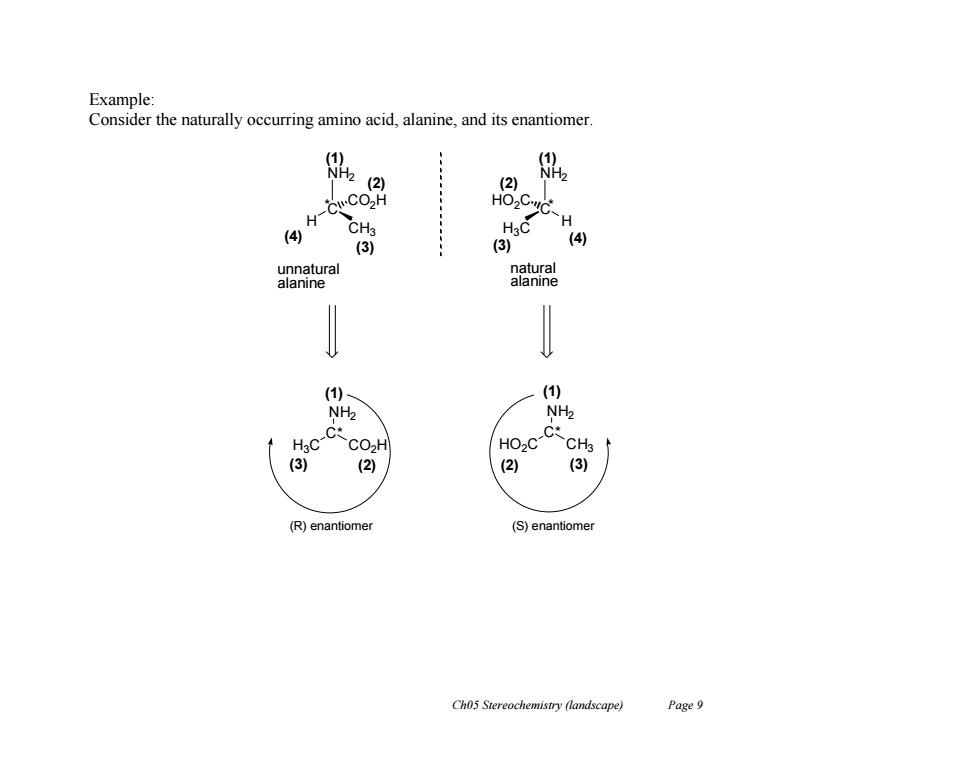
Example: Consider the naturally occurring amino acid,alanine,and its enantiomer (1 NH2 (2) 白 H CH3 H (4) (3) (3) (4) unnatural natural alanine alanine (1) (1) NH2 NH2 c-C CO2H HO2C-C CH3 (3) (2) ( (3) (R)enantiomer (S)enantiomer Ch05 Stereochemistry (landscape) Page 9
Ch05 Stereochemistry (landscape) Page 9 Example: Consider the naturally occurring amino acid, alanine, and its enantiomer. NH2 C H CO2H C* NH2 H3C CO2H * NH2 C H HO2C C* NH2 HO2C CH3 * (R) enantiomer (S) enantiomer CH3 H3C (1) (2) (3) (4) (1) (4) (2) (3) (1) (1) (3) (2) (2) (3) unnatural alanine natural alanine

Optical Activity Enantiomers have the same Boiling points Melting points Density. Almost all their physical properties are identical,but one exception is their effect on plane polarized light Enantiomeric compounds rotate the plane of polarized light in equal but opposite directions. Chiral compounds are called optically active compounds Optically active compounds which rotate plane polarized light clockwise (dextrorotatory)are designated (+)or d. Optically active compounds which rotate plane polarized light counterclockwise (levorotatory)are designated (- or I. Note:There is no relationship between(R)and(S)and the direction of the rotation of plane polarized light.(R) does not mean d and (+) (R)and(S)are just names from the CIP convention.(+)and(-)are experimentally observed physical properties. Ch05 Stereochemistry (landscape) Page 10
Ch05 Stereochemistry (landscape) Page 10 Optical Activity Enantiomers have the same Boiling points Melting points Density. Almost all their physical properties are identical, but one exception is their effect on plane polarized light. Enantiomeric compounds rotate the plane of polarized light in equal but opposite directions. Chiral compounds are called optically active compounds. Optically active compounds which rotate plane polarized light clockwise (dextrorotatory) are designated (+) or d. Optically active compounds which rotate plane polarized light counterclockwise (levorotatory) are designated (-) or l. Note: There is no relationship between (R) and (S) and the direction of the rotation of plane polarized light. (R) does not mean d and (+). (R) and (S) are just names from the CIP convention. (+) and (-) are experimentally observed physical properties