
ORGANIC CHEMISTRY Organic Chemistry I EIGHTH EDITION CHM 201 William A. Price, Ph.D. L.G.WADE,JR
Organic Chemistry I CHM 201 William A. Price, Ph.D
Introduction and Review: Structure and Bonding Atomic structure Lewis Structures Resonance Structural Formulas Acids and Bases
Introduction and Review: Structure and Bonding Atomic structure Lewis Structures Resonance Structural Formulas Acids and Bases
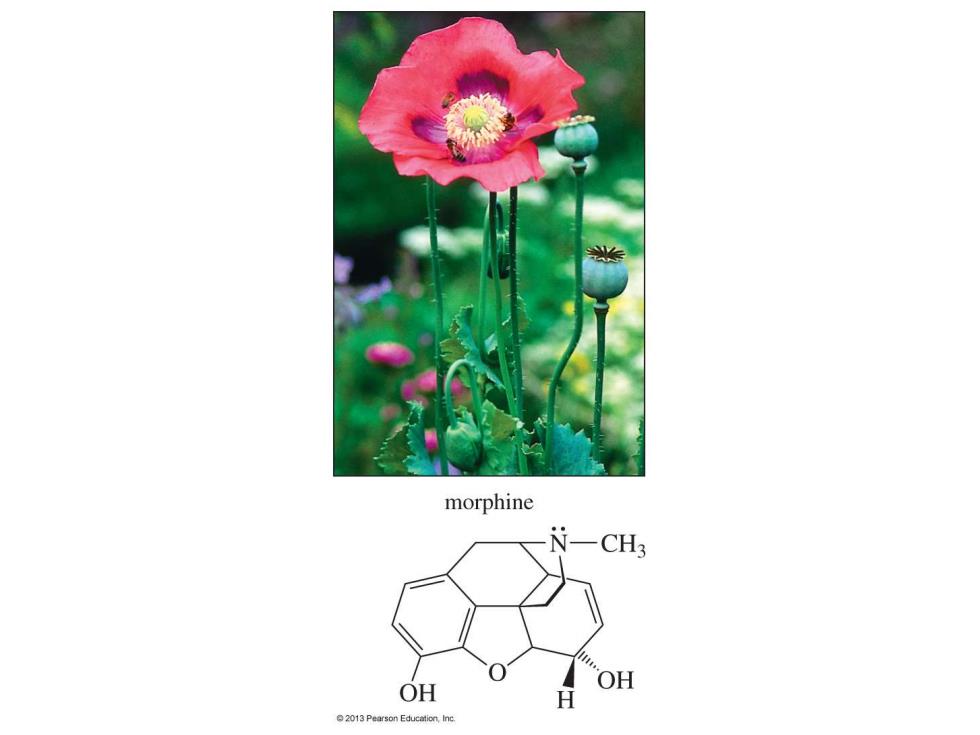
morphine N一CH3 OH OH 2013 Pearson Education,Inc
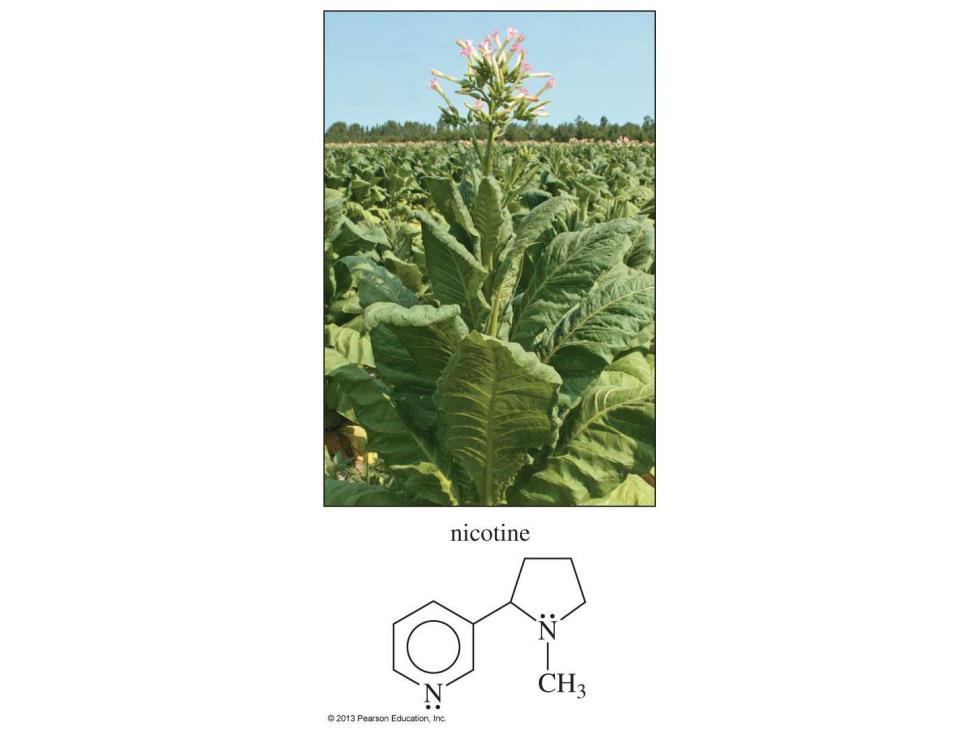
nicotine CH3

Electronic Structure of the Atom 。An atom has a dense, positively charged nucleus electron density surrounded by a cloud of electrons. The electron density is distance← distance from the nucleus highest at the nucleus and drops off exponentially with nucleus increasing distance from the nucleus in any direction. Chapter 1 5
Chapter 1 5 Electronic Structure of the Atom • An atom has a dense, positively charged nucleus surrounded by a cloud of electrons. • The electron density is highest at the nucleus and drops off exponentially with increasing distance from the nucleus in any direction
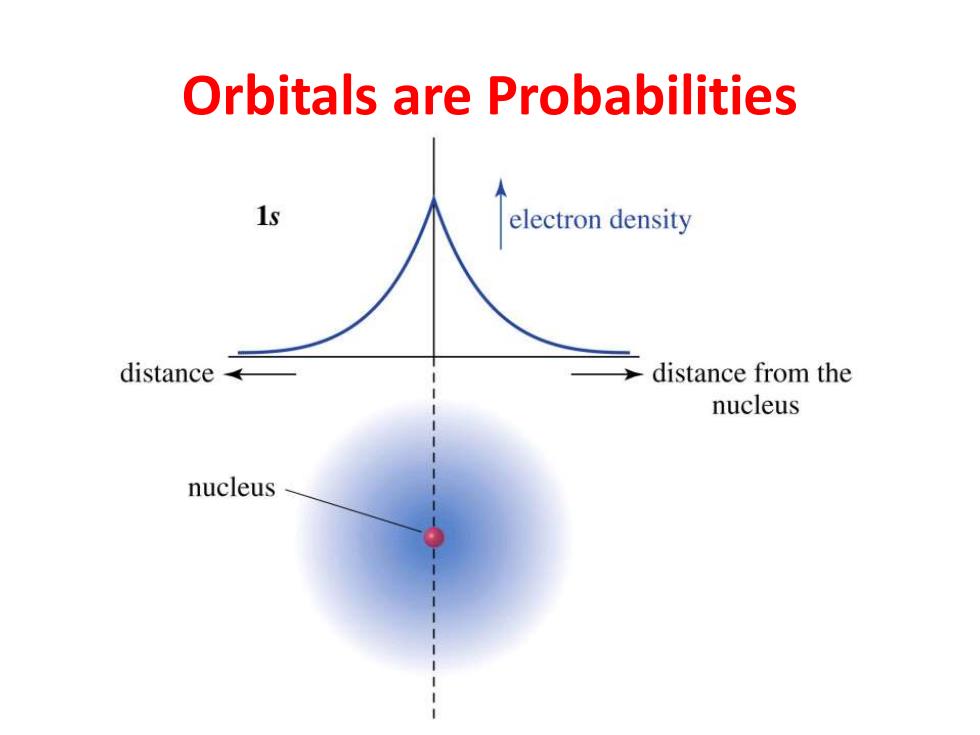
Orbitals are Probabilities 1s electron density distance →distance from the nucleus nucleus
Orbitals are Probabilities
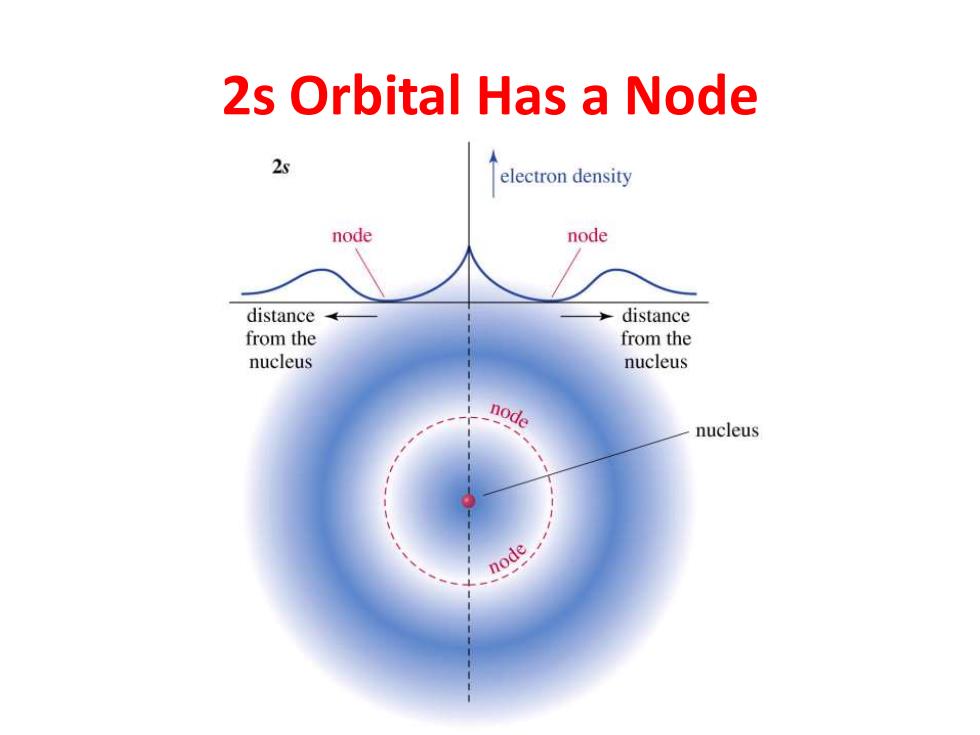
2s Orbital Has a Node 2s electron density node node distance distance from the from the nucleus nucleus node nucleus node
2s Orbital Has a Node
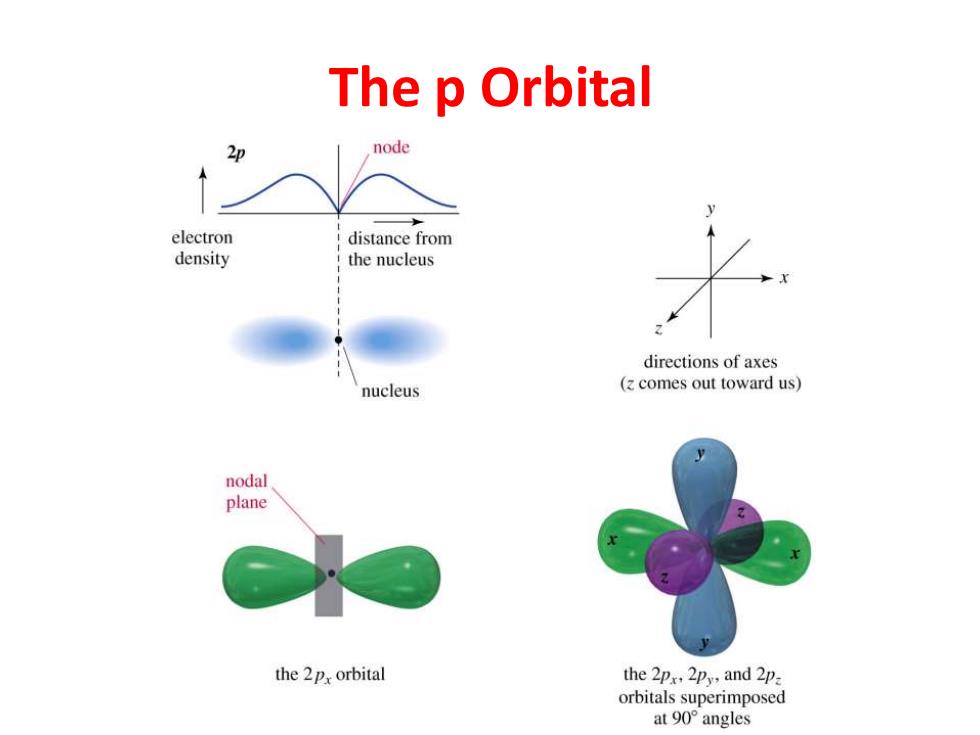
The p Orbital 2p node electron distance from density the nucleus directions of axes nucleus (z comes out toward us) nodal plane the 2px orbital the 2px,2py,and 2p orbitals superimposed at90°angles
The p Orbital
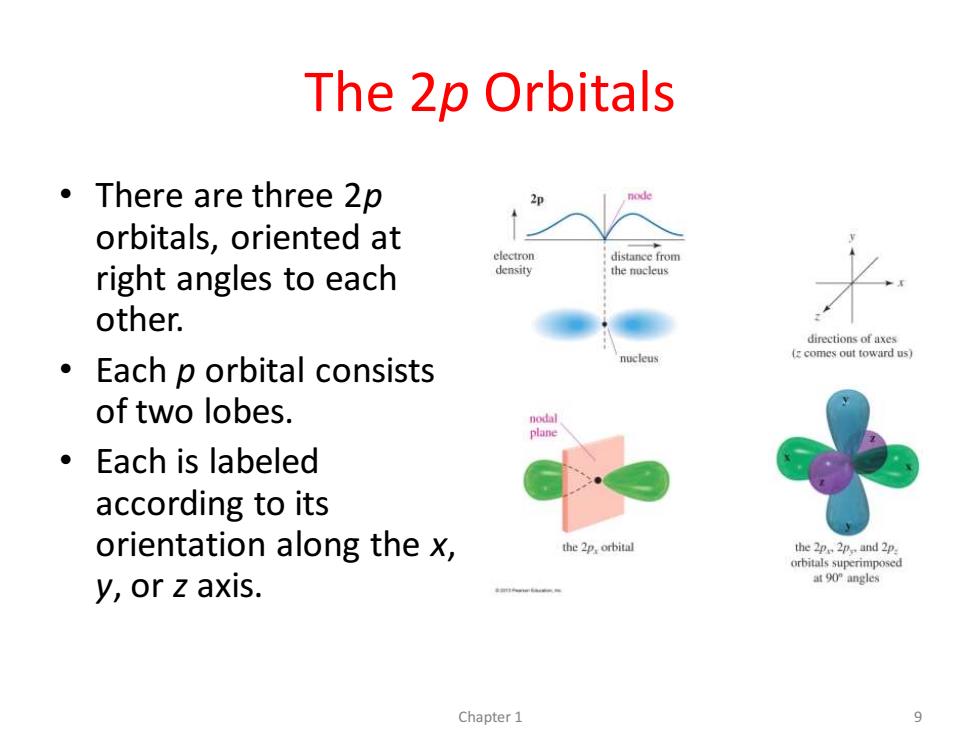
The 2p Orbitals ·There are three2p orbitals,oriented at electron distance from right angles to each density the nucleus other. directions of axes (zcomes out toward us) Each p orbital consists of two lobes. nodal 。Each is labeled according to its orientation along the x, the 2p,orbital theppand 2p orbitals superimposed y,or z axis. at 90"angles Chapter 1 9
The 2p Orbitals • There are three 2p orbitals, oriented at right angles to each other. • Each p orbital consists of two lobes. • Each is labeled according to its orientation along the x, y, or z axis. Chapter 1 9
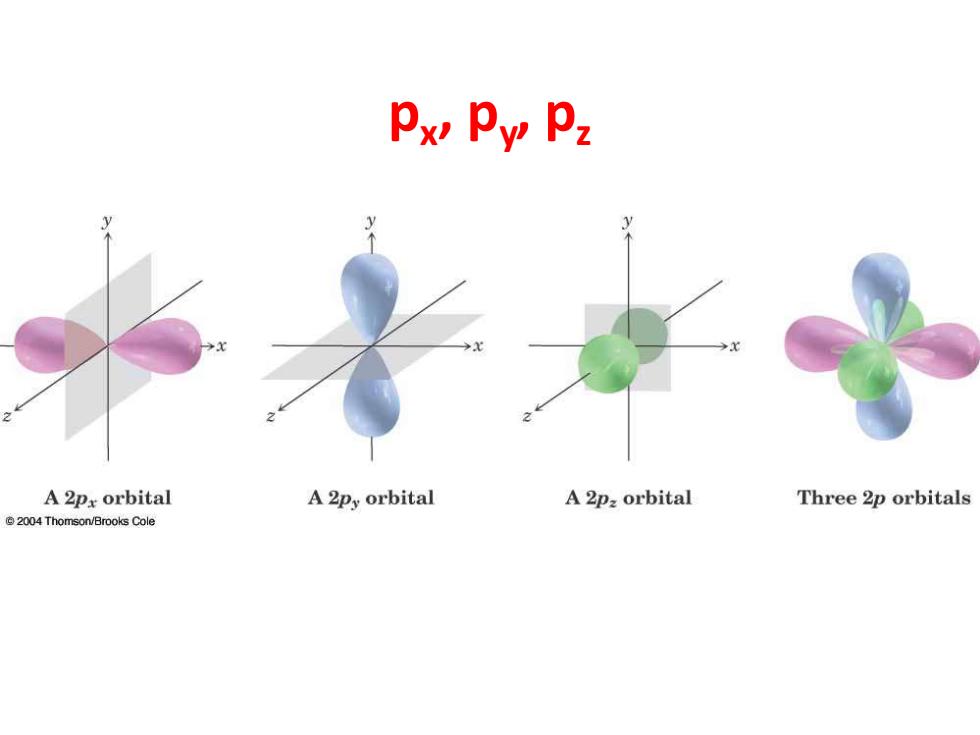
Px Py P2 A 2px orbital A 2py orbital A 2p:orbital Three 2p orbitals 2004 Thomson/Brooks Cole
px , py , pz