
Alkynes Reaction Acidity Synthesis
Alkynes Reaction Acidity Synthesis
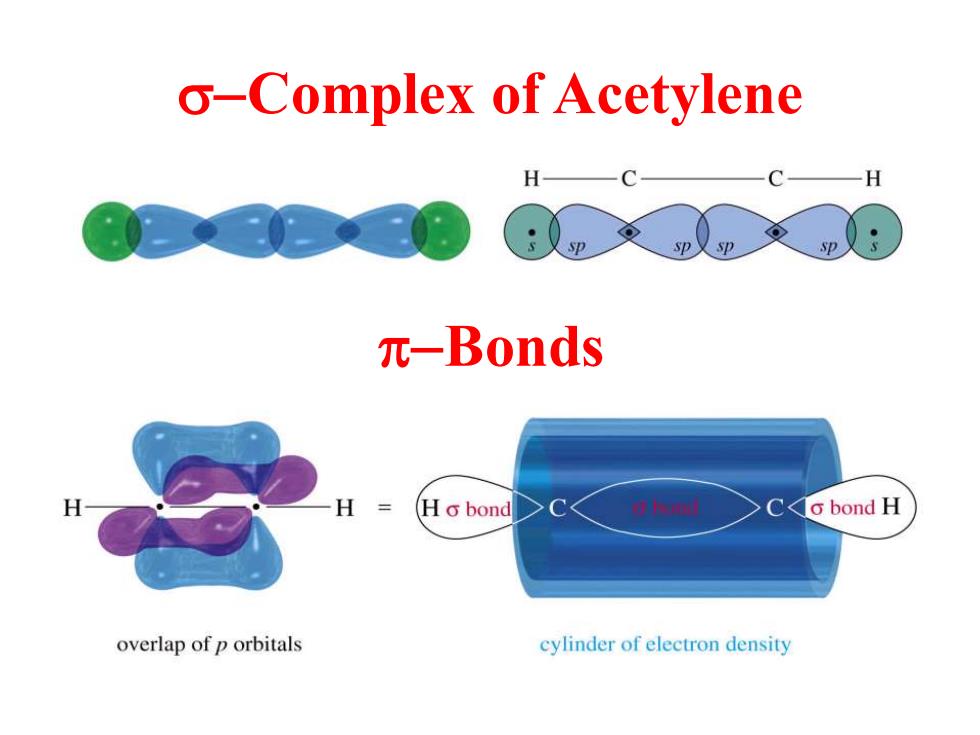
o-Complex of Acetylene H H 元-Bonds Ho bond o bond H overlap of p orbitals cylinder of electron density
s-Complex of Acetylene p-Bonds
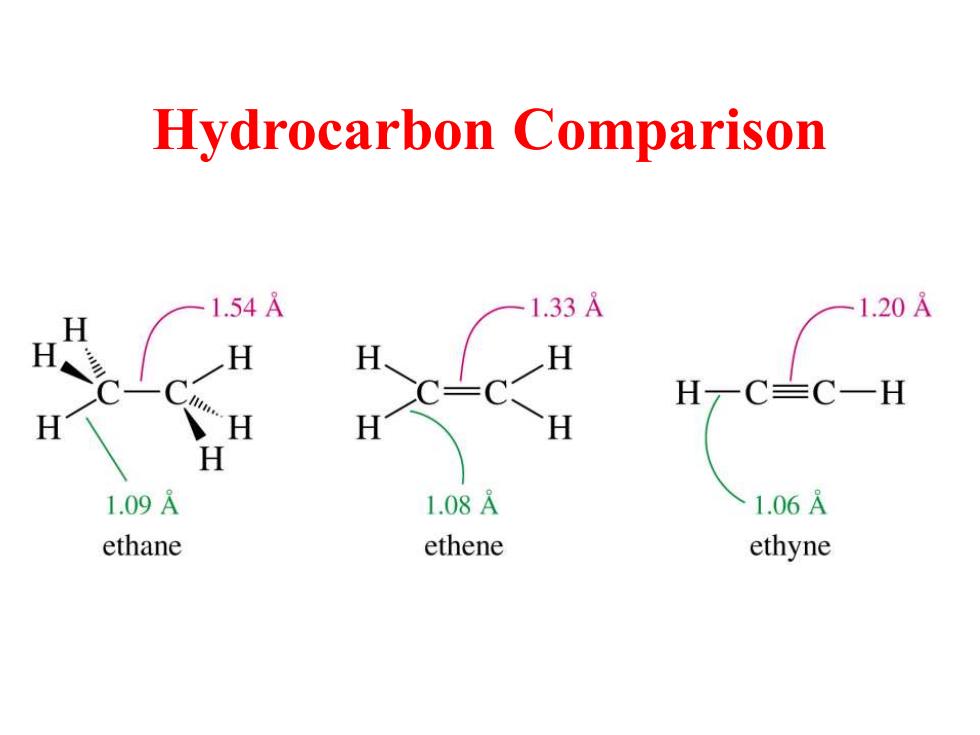
Hydrocarbon Comparison 1.54A 1.33A 1.20A H H一C=C一H H H 1.09A 1.08A 1.06A ethane ethene ethyne
Hydrocarbon Comparison
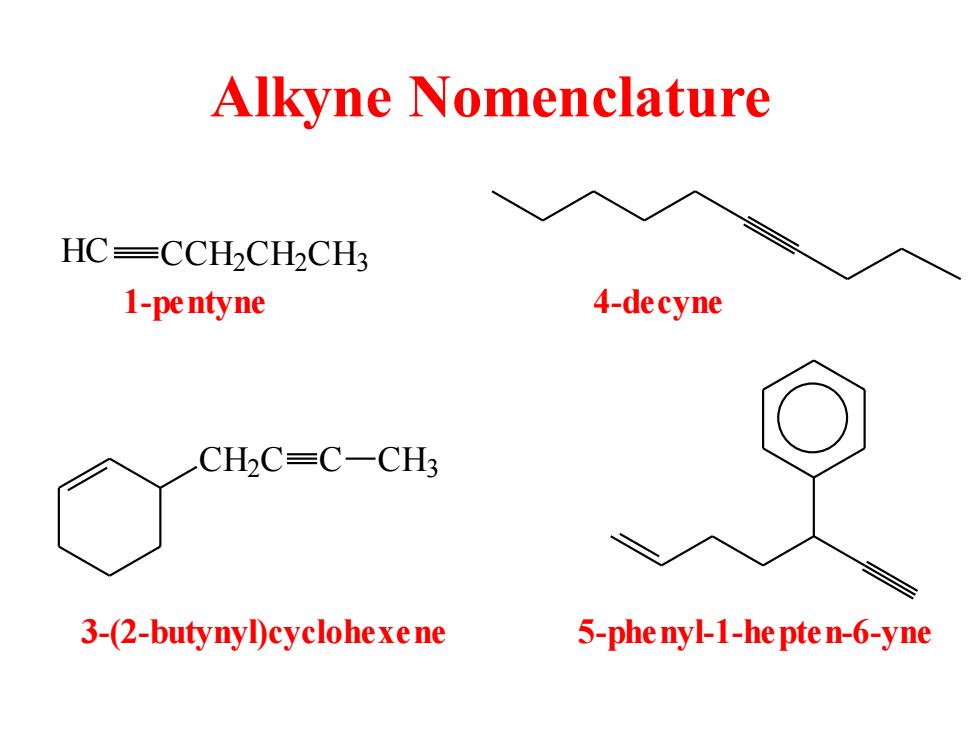
Alkyne Nomenclature HC=CCH2CH2CH3 1-pe ntyne 4-decyne CH2C=C-CH3 3-(2-butynyD)cyclohexe ne 5-phe nyl-1-he pte n-6-yne
Alkyne Nomenclature HC CCH2 CH2 CH3 1-pentyne 4-decyne CH2 C C CH3 3-(2-butynyl)cyclohexene 5-phenyl-1-hepten-6-yne
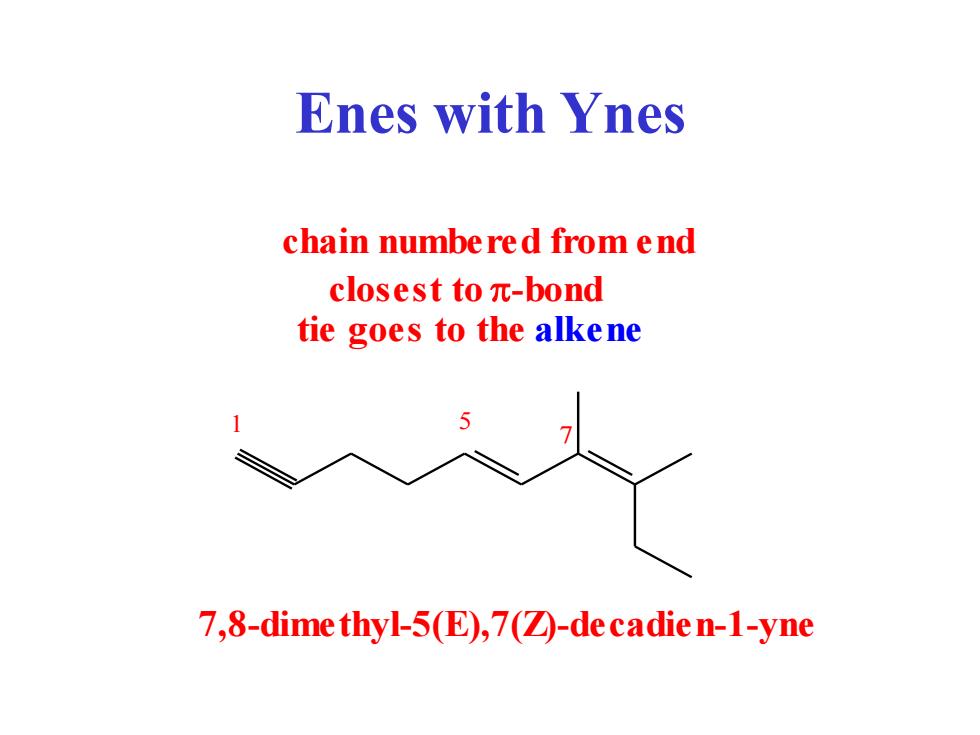
Enes with Ynes chain numbered from end closest toπ-bond tie goes to the alkene 7,8-dime thyl-5(E),7(2-de cadie n-1-yne
Enes with Ynes chain numbered from end closest to p-bond 1 5 7 7,8-dimethyl-5(E),7(Z)-decadien-1-yne tie goes to the alkene
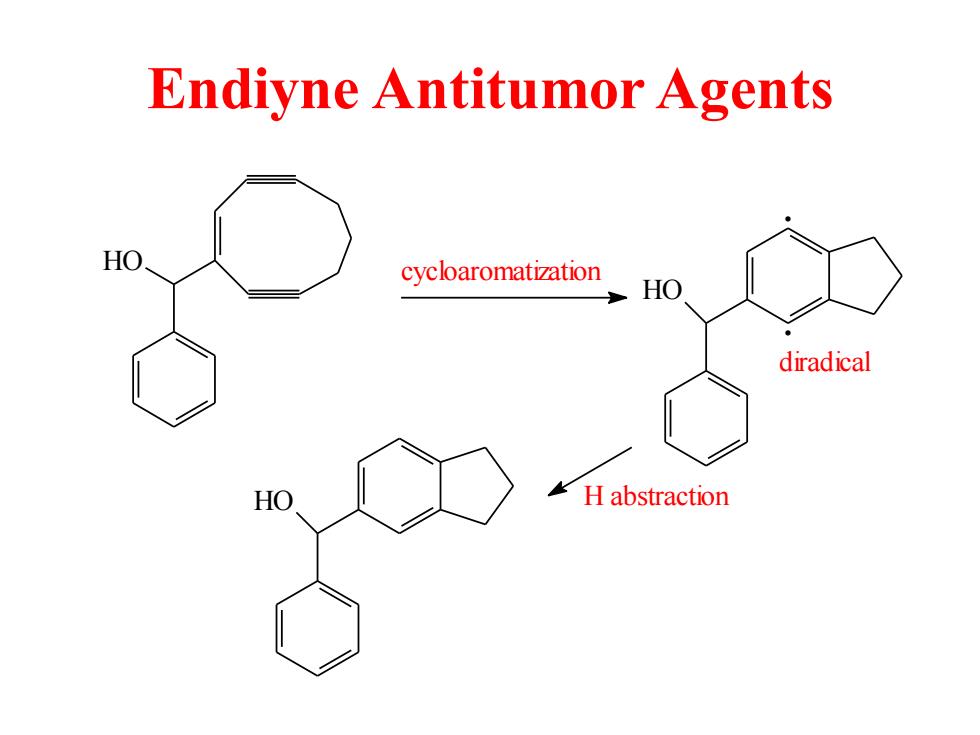
Endiyne Antitumor Agents cycloaromatization HO diradical HO H abstraction
Endiyne Antitumor Agents HO cycloaromatization HO . . HO H abstraction diradical
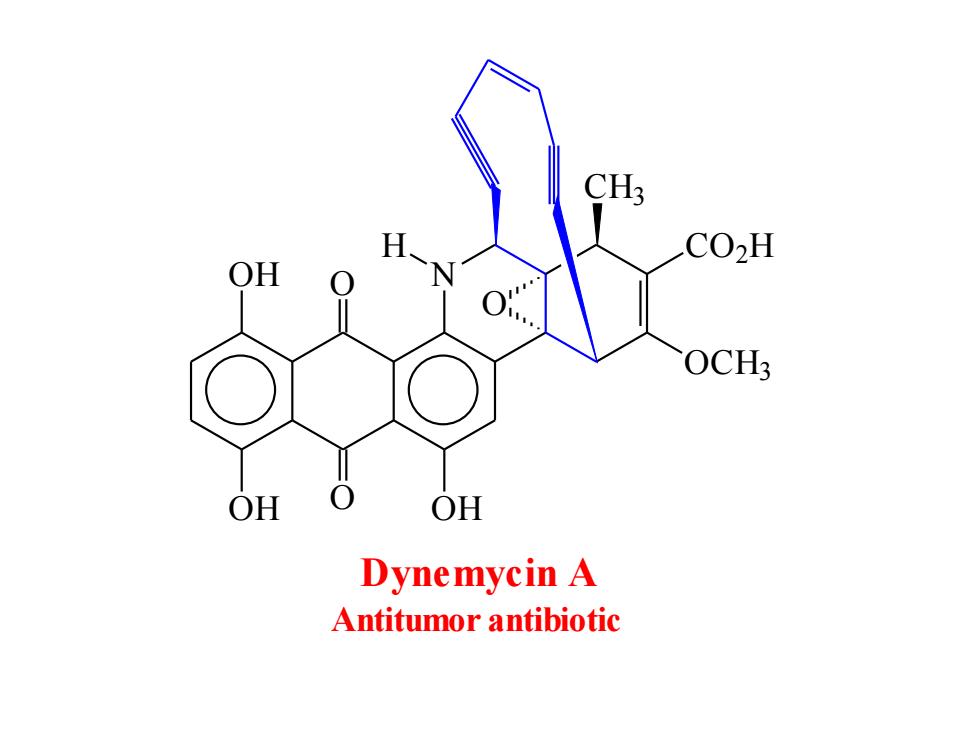
CH; CO2H OH OCH; OH 0 OH Dynemycin A Antitumor antibiotic
N H O O OH OH OH CO2 H OCH3 CH3 O Dynemycin A Antitumor antibiotic
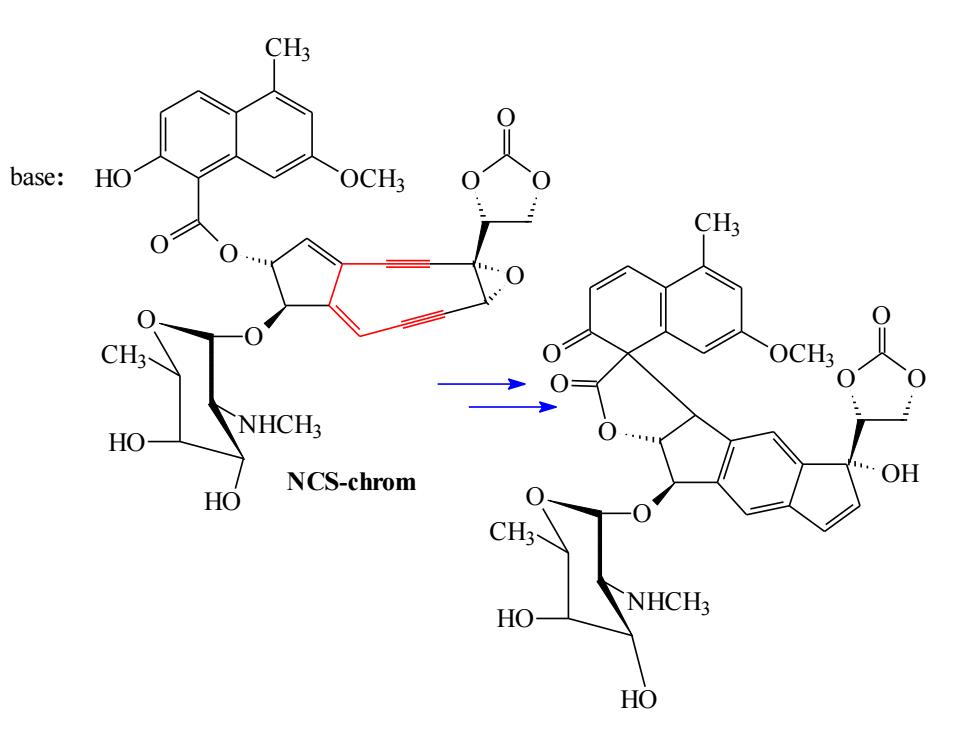
CH3 base: HO CH3 OCH3 NHCH3 HO NCS-chrom OH HO CH NHCH? HO HO
O O O O O CH3 HO OCH3 O O O NHCH3 HO HO CH3 base: O CH3 O OCH3 O O O NHCH3 HO HO CH3 O O O NCS-chrom OH
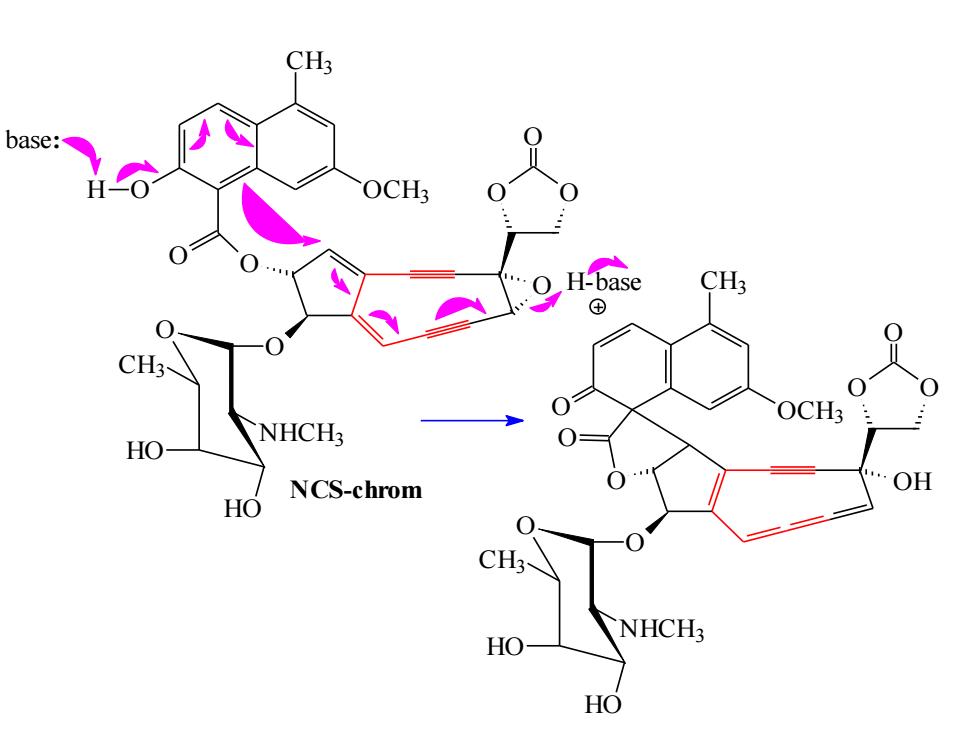
CH3 base: OCH3 H-base CH3 ⊕ NHCH: HO NCS-chrom OH HO CH3 NHCH HO HO
NCS-chrom O O O O O CH3 O OCH3 O O O NHCH3 HO HO CH3 H base: OH O O O O CH3 O OCH3 O O O NHCH3 HO HO CH3 H-base
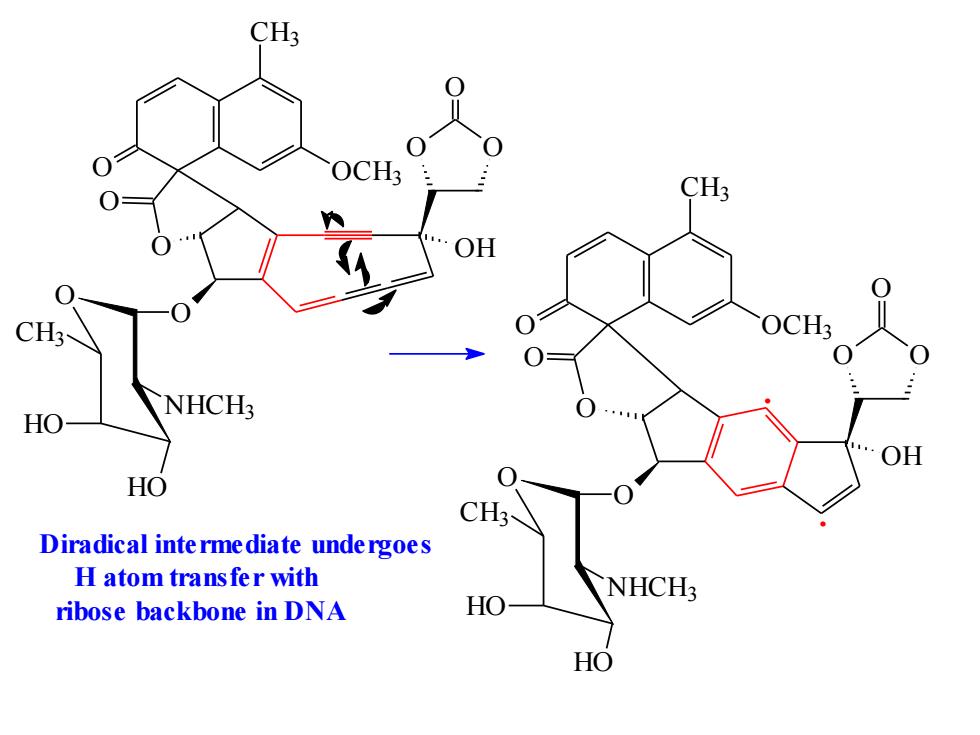
CH3 CH3 H OCH3 NHCH3 OH HO CH3 Diradical inte rmediate unde rgoes H atom transfer with NHCH3 ribose backbone in DNA HO HO
OH O O O O CH3 O OCH3 O O O NHCH3 HO HO CH3 O CH3 O OCH3 O O O NHCH3 HO HO CH3 O O O OH . . Diradical intermediate undergoes H atom transfer with ribose backbone in DNA