
Alkenes Addition Reactions Synthesis
Alkenes Addition Reactions Synthesis
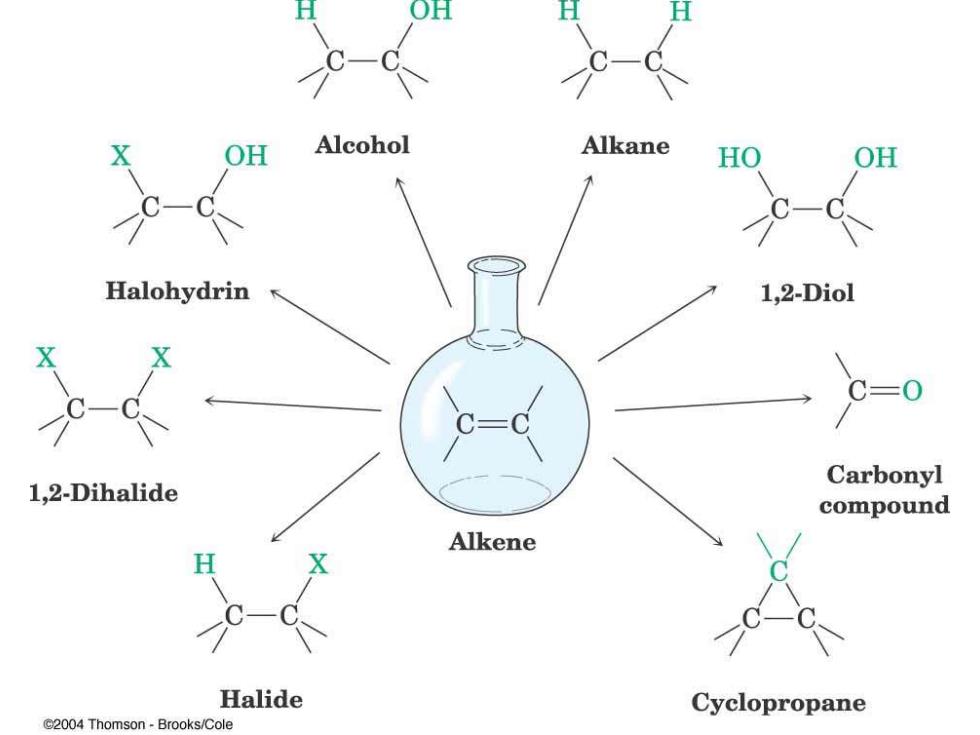
OH Alcohol Alkane HO OH Halohydrin 1,2-Diol C=0 Carbonyl 1,2-Dihalide compound Alkene H Halide Cyclopropane C2004 Thomson-Brooks/Cole
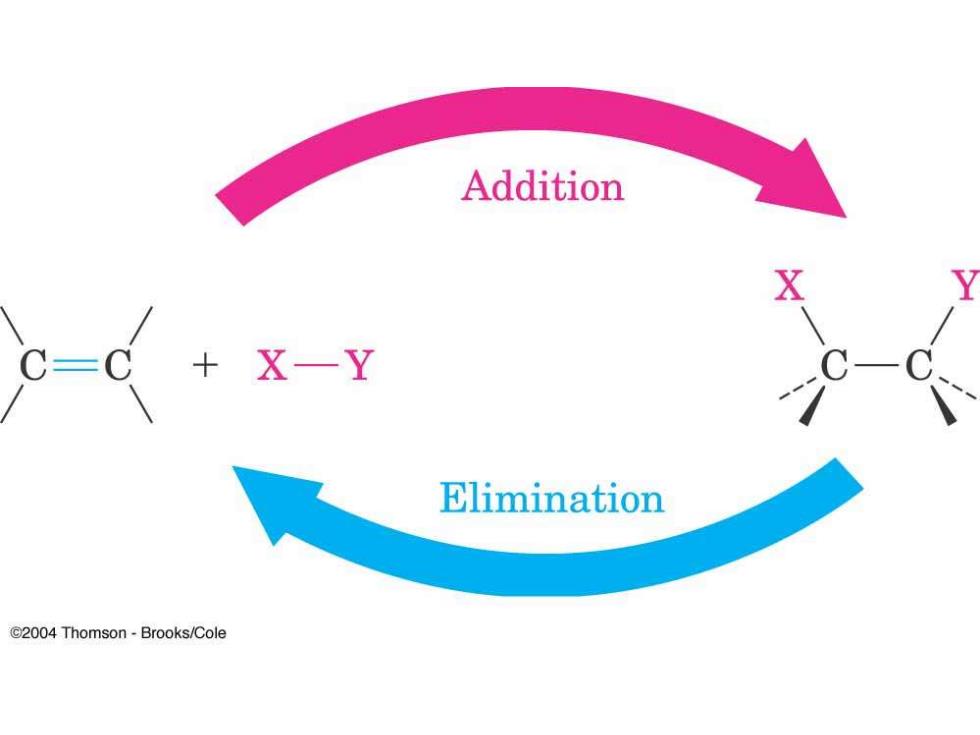
Addition X-Y Elimination C2004 Thomson-Brooks/Cole
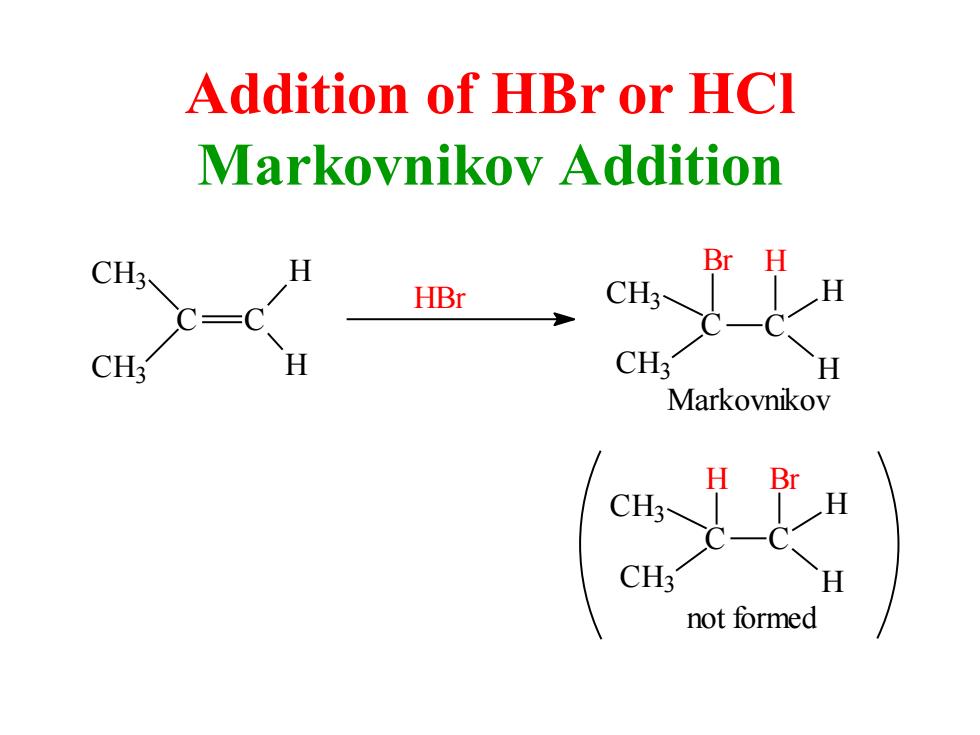
Addition of HBr or HCl Markovnikov Addition CH3 H Br H HBr CH3- H C=C CH: CH3 H Markovnikov H ● CH3 CH3 H not formed
Addition of HBr or HCl Markovnikov Addition Markovnikov Br C C H H H CH3 CH3 HBr CH3 CH3 C H H C CH3 CH3 C H H Br C H not formed
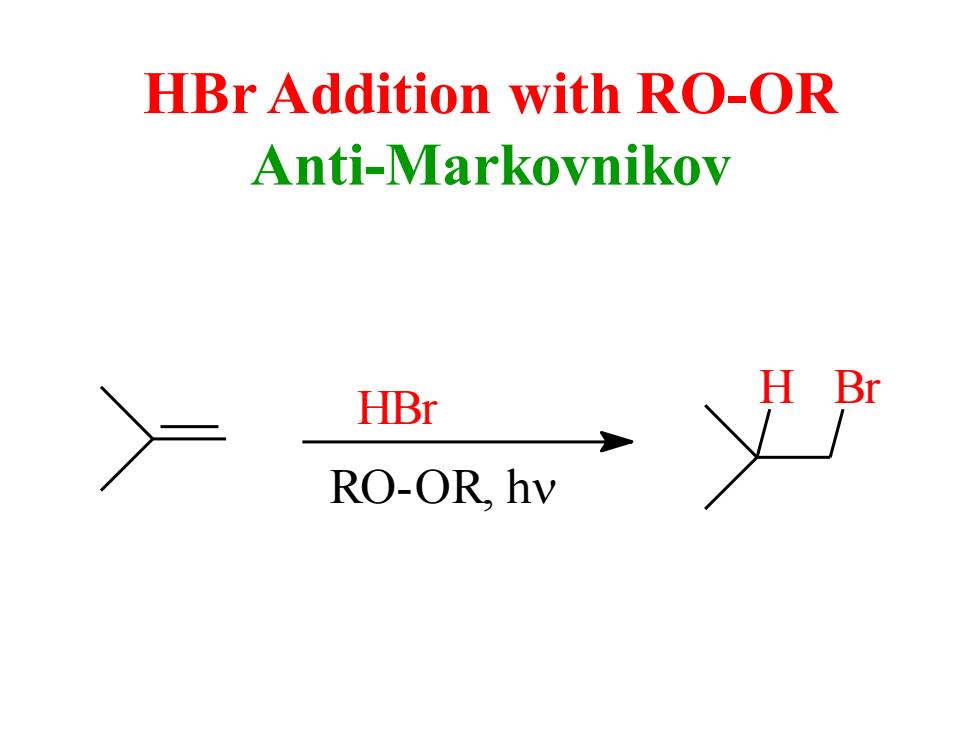
HBr Addition with RO-OR Anti-Markovnikov HBr Br RO-OR.hv
HBr Addition with RO-OR Anti-Markovnikov HBr RO-OR, h H Br

Free-Radical mechanism Initiation: RO-OR 2 RO' RO+HBr Br+ROH Propagation:i) +Br' H-Br Br
Free-Radical Mechanism Initiation: Propagation: i) ii) RO-OR 2 RO RO + HBr Br + ROH + Br Br Br + H-Br H Br + Br . . . . . .
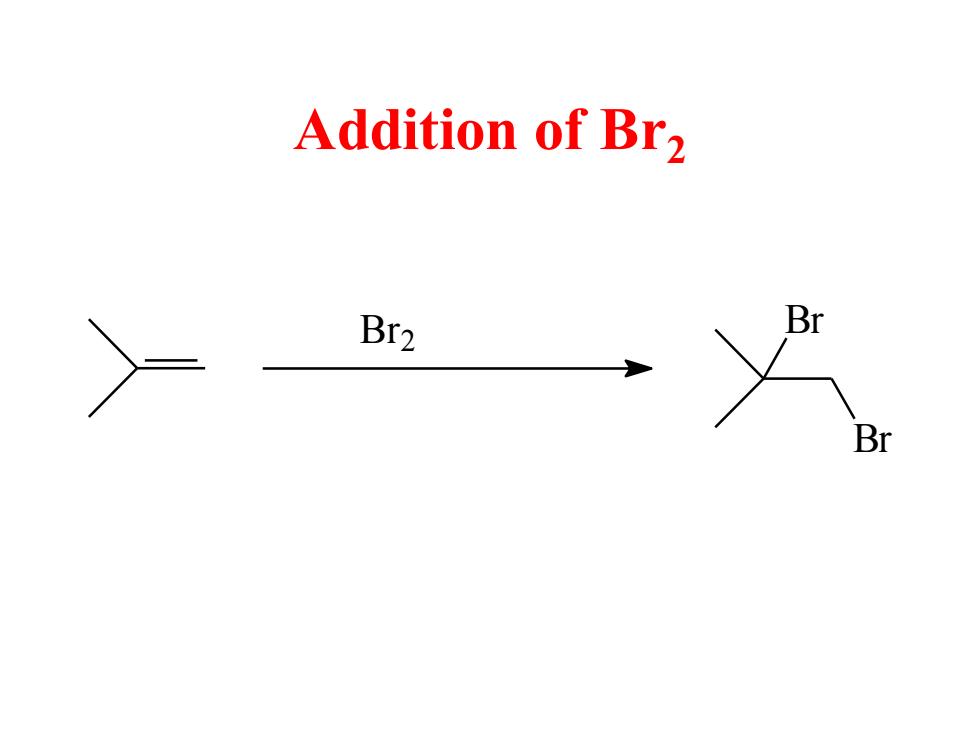
Addition of Br2 Br2 Br Br
Addition of Br2 Br Br Br2
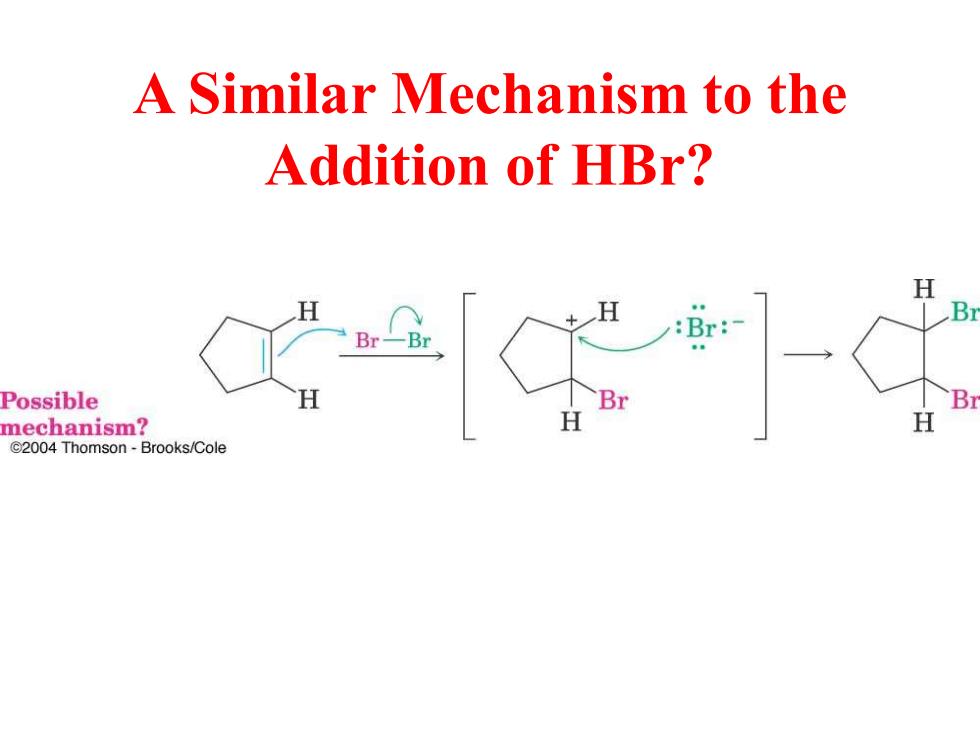
A Similar Mechanism to the Addition of HBr? H :Br: Br Possible Br mechanism? H H C2004 Thomson-Brooks/Cole
A Similar Mechanism to the Addition of HBr?
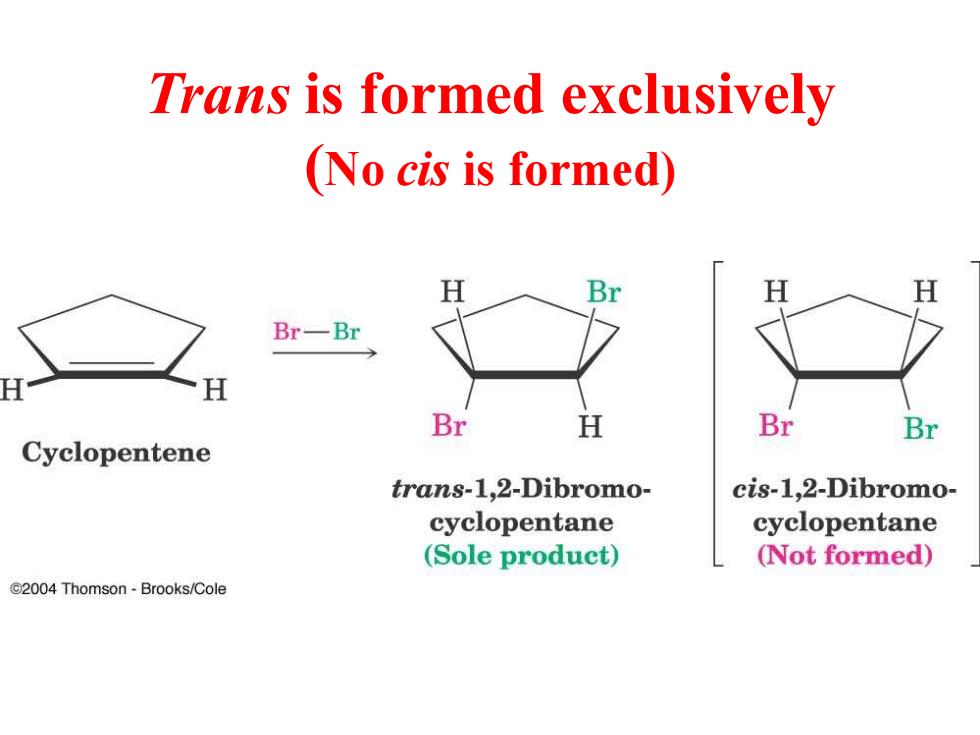
Trans is formed exclusively (No cis is formed) H Br H H Br-Br Br H Br Br Cyclopentene trans-1,2-Dibromo- cis-1,2-Dibromo- cyclopentane cyclopentane (Sole product) (Not formed) C2004 Thomson-Brooks/Cole
Trans is formed exclusively (No cis is formed)
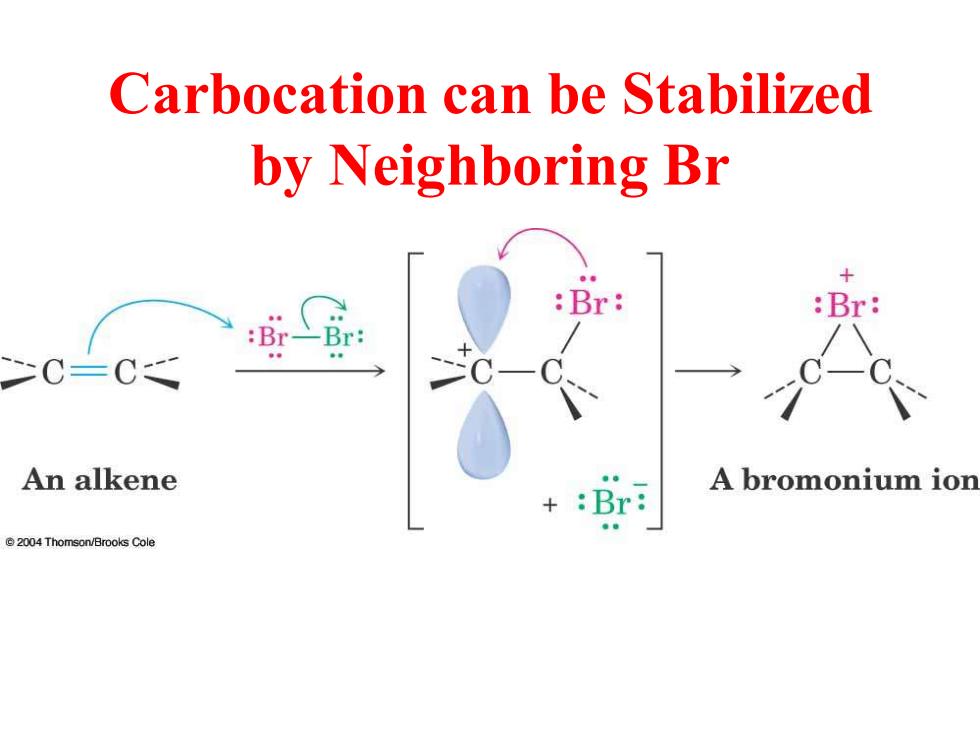
Carbocation can be Stabilized by Neighboring Br :Br: :Br: :Br-Br: C=C An alkene A bromonium ion :Br: 2004 Thomson/Brooks Cole
Carbocation can be Stabilized by Neighboring Br