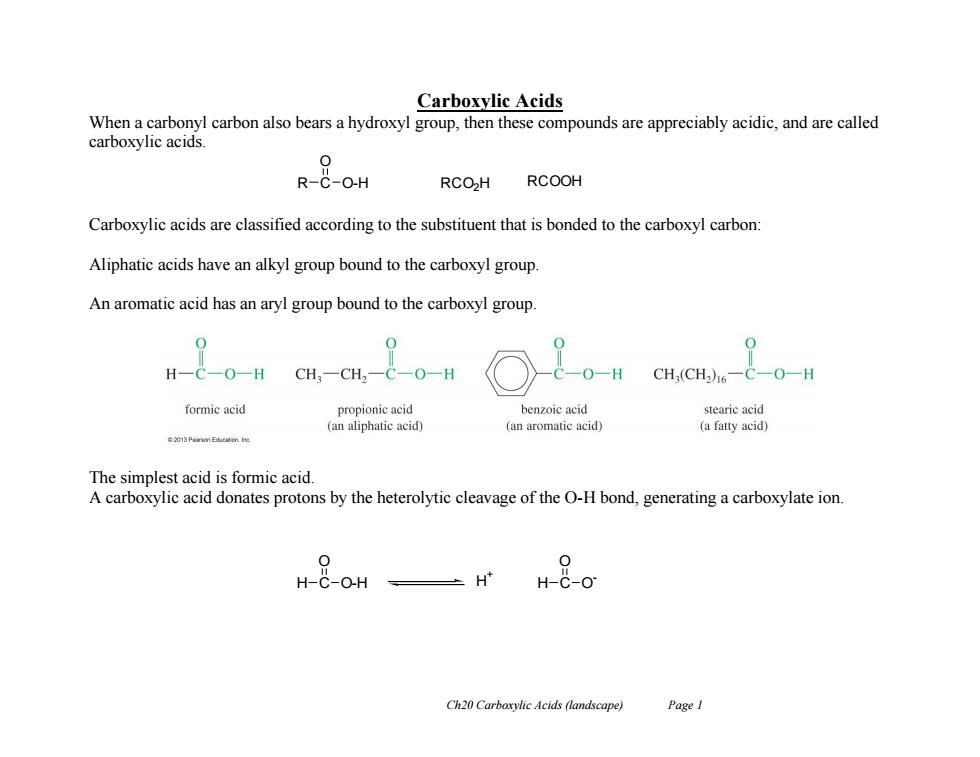
Carboxylic Acids When a carbonyl carbon also bears a hydroxyl group,then these compounds are appreciably acidic,and are called carboxylic acids. 0 R-C-O-H RCOH RCOOH Carboxylic acids are classified according to the substituent that is bonded to the carboxyl carbon: Aliphatic acids have an alkyl group bound to the carboxyl group. An aromatic acid has an aryl group bound to the carboxyl group 0 H-C-O-H CH:-CH,-C- -O-H C一O-H CH,(CH)6一C-O-H formic acid propionic acid benzoic acid stearic acid (an aliphatic acid) (an aromatic acid) (a fatty acid) ◆20aP3 Edicaton he The simplest acid is formic acid. A carboxylic acid donates protons by the heterolytic cleavage of the O-H bond,generating a carboxylate ion 0 H-C-O-H H H-C-O Ch20 Carboxylic Acids (landscape) PageI
Ch20 Carboxylic Acids (landscape) Page 1 Carboxylic Acids When a carbonyl carbon also bears a hydroxyl group, then these compounds are appreciably acidic, and are called carboxylic acids. Carboxylic acids are classified according to the substituent that is bonded to the carboxyl carbon: Aliphatic acids have an alkyl group bound to the carboxyl group. An aromatic acid has an aryl group bound to the carboxyl group. The simplest acid is formic acid. A carboxylic acid donates protons by the heterolytic cleavage of the O-H bond, generating a carboxylate ion. R C O O-H RCO2H RCOOH H C O O-H H C O O H + -
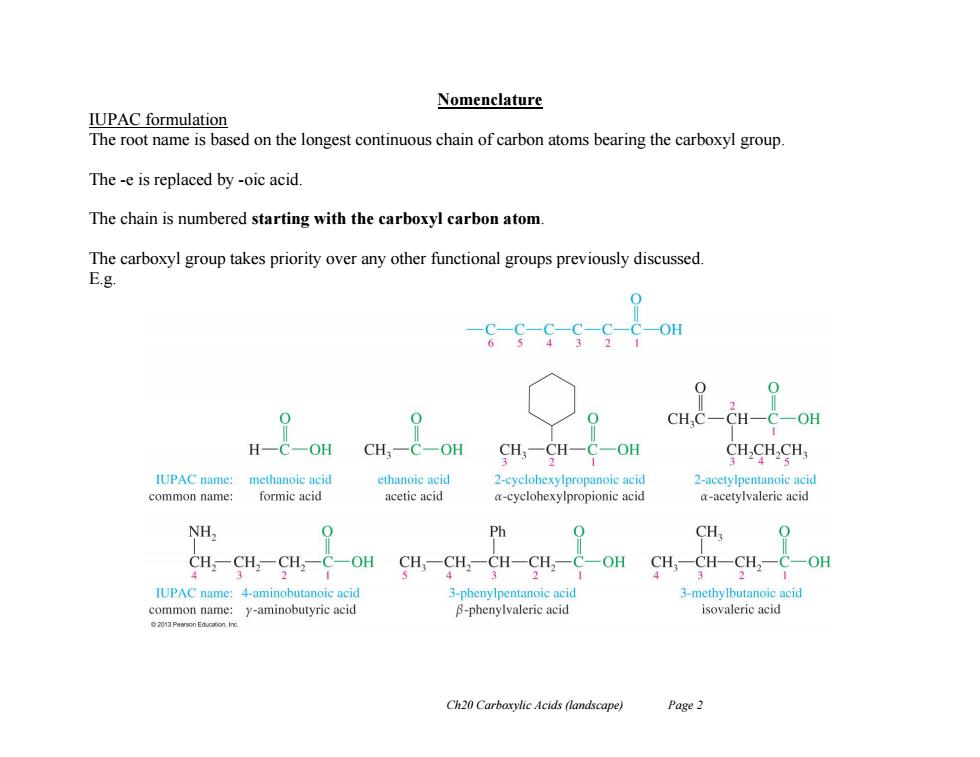
Nomenclature IUPAC formulation The root name is based on the longest continuous chain of carbon atoms bearing the carboxyl group. The -e is replaced by -oic acid The chain is numbered starting with the carboxyl carbon atom The carboxyl group takes priority over any other functional groups previously discussed. E.g. c-s-G6-s c CH,C-CH-C-OH H一C-OH CH,-C-OH CH3-CH-C-OH CH,CH,CH IUPAC name: methanoic acid ethanoic acid 2-cyclohexylpropanoic acid 2-acetylpentanoic acid common name: formic acid acetic acid a-cyclohexylpropionic acid a-acetylvaleric acid NH, Ph CH, -OH CH,-CH,一CH-CH2-C-OH CH-CH-H,一 -OH IUPAC name:4-aminobutanoic acid 3-phenylpentanoic acid 3-methylbutanoic acid common name:y-aminobutyric acid B-phenylvaleric acid isovaleric acid 2013 Peorson Educooon Inc Ch20 Carboxylic Acids (landscape) Page 2
Ch20 Carboxylic Acids (landscape) Page 2 Nomenclature IUPAC formulation The root name is based on the longest continuous chain of carbon atoms bearing the carboxyl group. The -e is replaced by -oic acid. The chain is numbered starting with the carboxyl carbon atom. The carboxyl group takes priority over any other functional groups previously discussed. E.g
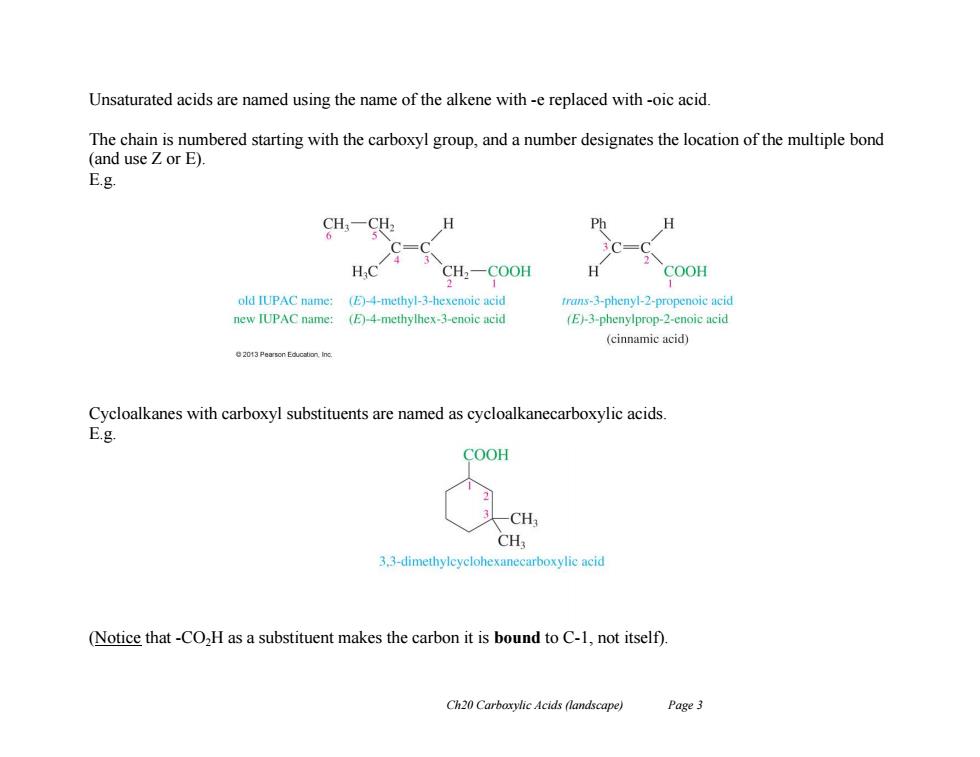
Unsaturated acids are named using the name of the alkene with -e replaced with -oic acid. The chain is numbered starting with the carboxyl group,and a number designates the location of the multiple bond (and use Z or E). E.g. P CH2-COOH COOH old IUPAC name:()-4-methyl-3-hexenoic acid trans-3-phenyl-2-propenoic acid new IUPAC name: (E)-4-methylhex-3-enoic acid (E)-3-phenylprop-2-enoic acid (cinnamic acid) 20t3 Pearson Educslion inc. Cycloalkanes with carboxyl substituents are named as cycloalkanecarboxylic acids. E.g COOH -CH CH 3.3-dimethyleyclohexanecarboxylic acid (Notice that -CO,H as a substituent makes the carbon it is bound to C-1,not itself). Ch20 Carboxylic Acids (landscape) Page 3
Ch20 Carboxylic Acids (landscape) Page 3 Unsaturated acids are named using the name of the alkene with -e replaced with -oic acid. The chain is numbered starting with the carboxyl group, and a number designates the location of the multiple bond (and use Z or E). E.g. Cycloalkanes with carboxyl substituents are named as cycloalkanecarboxylic acids. E.g. (Notice that -CO2H as a substituent makes the carbon it is bound to C-1, not itself)
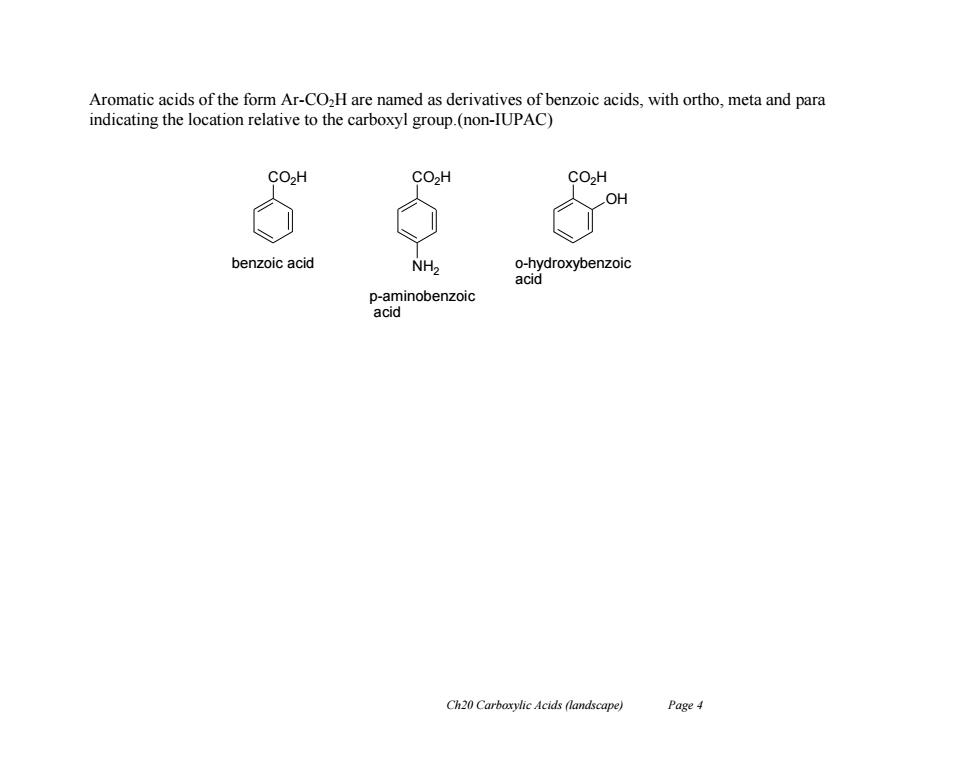
Aromatic acids of the form Ar-CO2H are named as derivatives of benzoic acids,with ortho,meta and para indicating the location relative to the carboxyl group.(non-IUPAC) CO2H CO2H CO>H OH benzoic acid NH> o-hydroxybenzoic acid p-aminobenzoic acid Ch20 Carboxylic Acids (landscape) Page 4
Ch20 Carboxylic Acids (landscape) Page 4 Aromatic acids of the form Ar-CO2H are named as derivatives of benzoic acids, with ortho, meta and para indicating the location relative to the carboxyl group.(non-IUPAC) CO2H CO2H CO2H benzoic acid p-aminobenzoic acid o-hydroxybenzoic acid NH2 OH
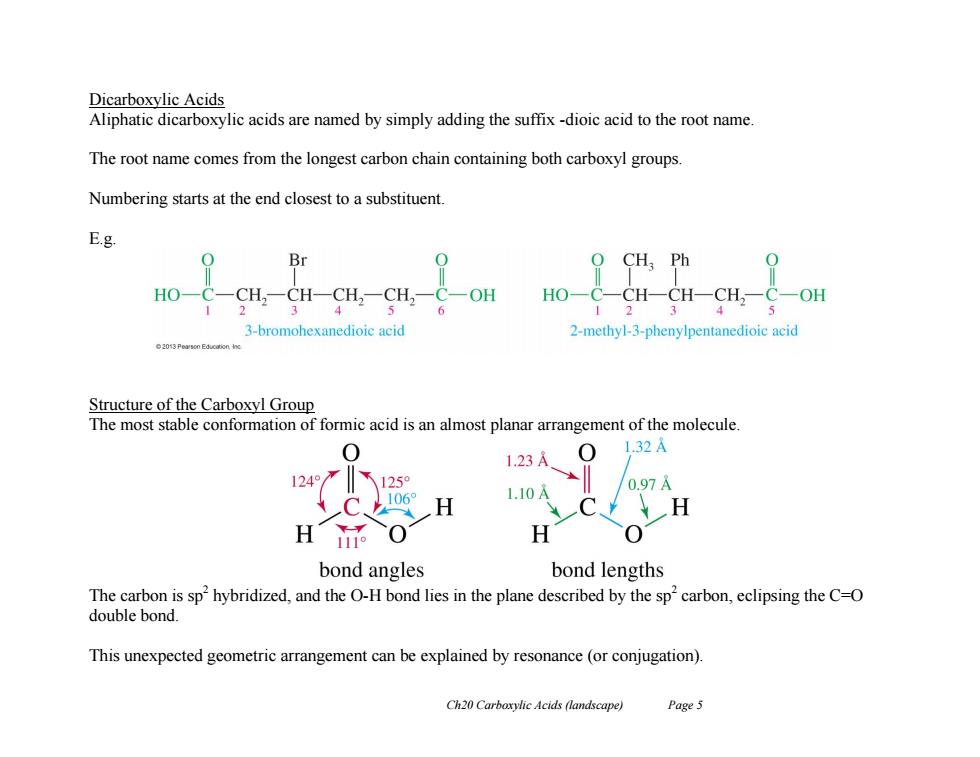
Dicarboxylic Acids Aliphatic dicarboxylic acids are named by simply adding the suffix -dioic acid to the root name. The root name comes from the longest carbon chain containing both carboxyl groups. Numbering starts at the end closest to a substituent. E.g. Br O CH Ph HO-C-CH2-CH-CH,-CH,-C-OH 4 HO-C一SH-CH-CH,-C-OH 1 3-bromohexanedioic acid 2-methyl-3-phenylpentanedioic acid Structure of the Carboxyl Group The most stable conformation of formic acid is an almost planar arrangement of the molecule. 0 1.32A 1.23A 1245125 1.10A 0.97A 1106° H H 0 bond angles bond lengths The carbon is sp2hybridized,and the O-H bond lies in the plane described by the sp'carbon,eclipsing the C=O double bond. This unexpected geometric arrangement can be explained by resonance(or conjugation). Ch20 Carboxylic Acids (landscape) Page 5
Ch20 Carboxylic Acids (landscape) Page 5 Dicarboxylic Acids Aliphatic dicarboxylic acids are named by simply adding the suffix -dioic acid to the root name. The root name comes from the longest carbon chain containing both carboxyl groups. Numbering starts at the end closest to a substituent. E.g. Structure of the Carboxyl Group The most stable conformation of formic acid is an almost planar arrangement of the molecule. The carbon is sp2 hybridized, and the O-H bond lies in the plane described by the sp2 carbon, eclipsing the C=O double bond. This unexpected geometric arrangement can be explained by resonance (or conjugation)

Three resonance forms can be written for formic acid. 0 Q 0- H-C-O-H →H-C0H H-CtO-H major minor very minor The second structure requires the C-O-H bonds to be co-planar. One of the unshared lone pairs of oxygen is delocalized into the electrophilic system of the carbonyl group (One of the lone pairs on the hydroxyl oxygen is conjugated with the C=O double bond). Acidity Carboxylic acids can dissociate in aqueous solution into carboxylate ions and protons The equilibrium constant for this process is Ka,and more frequently we talk in terms of pK O-H+H,OR -0-+H,0 K= [R一CO2]H,O [R-CO,H] pK -l0g10 K Ch20 Carboxylic Acids (landscape) Page 6
Ch20 Carboxylic Acids (landscape) Page 6 Three resonance forms can be written for formic acid. The second structure requires the C-O-H bonds to be co-planar. One of the unshared lone pairs of oxygen is delocalized into the electrophilic system of the carbonyl group. (One of the lone pairs on the hydroxyl oxygen is conjugated with the C=O double bond). Acidity Carboxylic acids can dissociate in aqueous solution into carboxylate ions and protons. The equilibrium constant for this process is Ka , and more frequently we talk in terms of pKa . H C O O H H C O O- H H C O O- H + + major minor very minor
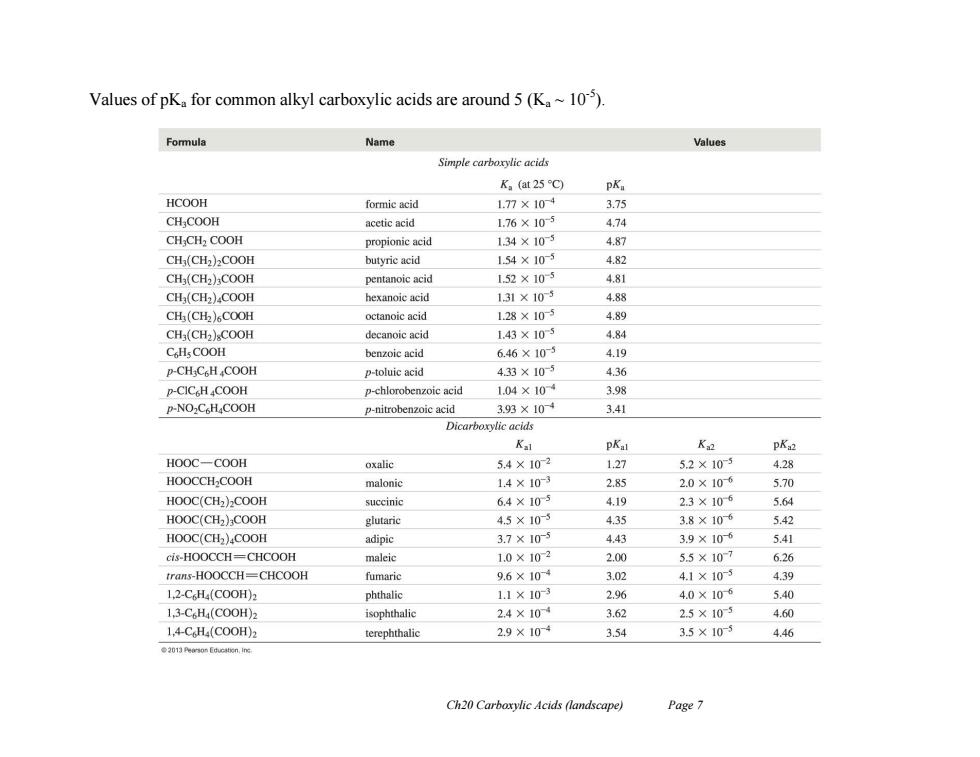
Values of pK for common alkyl carboxylic acids are around 5(K10). Formula Name Values Simple carboxylic acids K。(at25C) pKa HCOOH formic acid 1.77×104 3.75 CH;COOH acetic acid 1,76×105 474 CH:CH2 COOH propionic acid 1.34×105 4.87 CH(CH2)2COOH butyric acid 154×105 4.82 CH3(CH2)COOH pentanoic acid 1.52×105 4.81 CH3(CH2)4COOH hexanoic acid 131×105 4.88 CH (CH2)COOH octanoic acid 1.28×105 4.89 CH(CH2)COOH decanoic acid 1.43×105 4.84 CHsCOOH benzoic acid 6.46×10 4.19 P-CH;CsH COOH p-toluic acid 433×105 4.36 p-CIC HCOOH p-chlorobenzoic acid 1.04×104 3.98 P-NO2C H4COOH p-nitrobenzoic acid 3.93×104 3.41 Dicarboxylic acids Kal pKal K pK32 HOOC一COOH oxalic 5.4×102 1.27 5.2×105 4.28 HOOCCH2COOH malonic 1.4×103 2.85 2.0×106 5.70 HOOC(CH2)COOH succinic 6.4×105 4.19 2.3×106 5.64 HOOC(CH2)COOH glutaric 4.5×105 4.35 3.8×106 5.42 HOOC(CH2)COOH adipic 3.7×105 4.43 3.9×106 5.41 cis-HOOCCH=CHCOOH maleic 1.0×102 2.00 55×107 6.26 trans-HOOCCH=CHCOOH fumaric 9.6×104 3.02 4.1×103 4.39 1,2-C6H(C0OH)2 phthalic 1.1×103 2.96 4.0×106 540 1,3-C6H(C00H)2 isophthalie 2.4×10 3.62 2.5×105 4.60 1,4-CH(C00H)2 terephthalic 2.9×10 3.54 3.5×105 4.46 Ch20 Carboxylic Acids (landscape) Page 7
Ch20 Carboxylic Acids (landscape) Page 7 Values of pKa for common alkyl carboxylic acids are around 5 (Ka ~ 10-5 )

E.g.ethanoic acid has pK=4.74,(alcohols have pK~16,so carboxylic acids are about 10 times more acidic than alcohols). The reason why carboxylic acids are much more acidic than alcohols is because the carboxylate anion is much more stable than the alkoxide anion. Both alcohols and carboxylic acids are acidic since their respective O-H bonds can be broken heterolytically, giving a proton and an oxygen anion. R一0-H+H,δ:÷ R-6: +H,0冰6 (K,=10-16 alcohol alkoxide K5 R -C-0-H+H,ǒ:。→ R →R H.O+ (K≡105 acid carboxylate R-0- +HO+ R-OH +H2O 0 R-C HO+ R-COOH H2O stabilization carboxylate The difference lies in the fact that the carboxylate anion has the negative charge spread out over two oxygen atoms, whereas the alcohol has the negative charge localized on a single oxygen atom. Ch20 Carboxylic Acids (landscape) Page 8
Ch20 Carboxylic Acids (landscape) Page 8 E.g. ethanoic acid has pKa = 4.74, (alcohols have pKa ~ 16, so carboxylic acids are about 1011 times more acidic than alcohols). The reason why carboxylic acids are much more acidic than alcohols is because the carboxylate anion is much more stable than the alkoxide anion. Both alcohols and carboxylic acids are acidic since their respective O-H bonds can be broken heterolytically, giving a proton and an oxygen anion. The difference lies in the fact that the carboxylate anion has the negative charge spread out over two oxygen atoms, whereas the alcohol has the negative charge localized on a single oxygen atom

The carboxylate anion can be viewed as a resonance hybrid of the two anionic structures,or as a conjugated system of three interacting p orbitals containing four electrons(like the allylic anion system). 0 02 The C and two oxygens are all sp'hybridized,and the remaining p orbitals create the nt MO system giving rise to the half t bond between each C and O,and the half negative charge on the end oxygens. Dicarboxylic Acids These have two dissociation constants,since they can lose two protons. HO CH2 OH OH 0 CH, malonic acid KL=1.4×10-3 anion K2=2.0×10-6 dianion +2H20 +H30t+H20 +2HO+ 2013 he K for the first dissociation and K2 for the second dissociation,which generates a dianion. K2 is always less than K(the second carboxyl group is less acidic)since it takes extra energy to overcome the second negative charge being so close to the first negative charge. Ch20 Carboxylic Acids (landscape) Page 9
Ch20 Carboxylic Acids (landscape) Page 9 The carboxylate anion can be viewed as a resonance hybrid of the two anionic structures, or as a conjugated system of three interacting p orbitals containing four electrons (like the allylic anion system). The C and two oxygens are all sp2 hybridized, and the remaining p orbitals create the MO system giving rise to the half bond between each C and O, and the half negative charge on the end oxygens. Dicarboxylic Acids These have two dissociation constants, since they can lose two protons. Ka1 for the first dissociation and Ka2 for the second dissociation, which generates a dianion. Ka2 is always less than Ka1 (the second carboxyl group is less acidic) since it takes extra energy to overcome the second negative charge being so close to the first negative charge
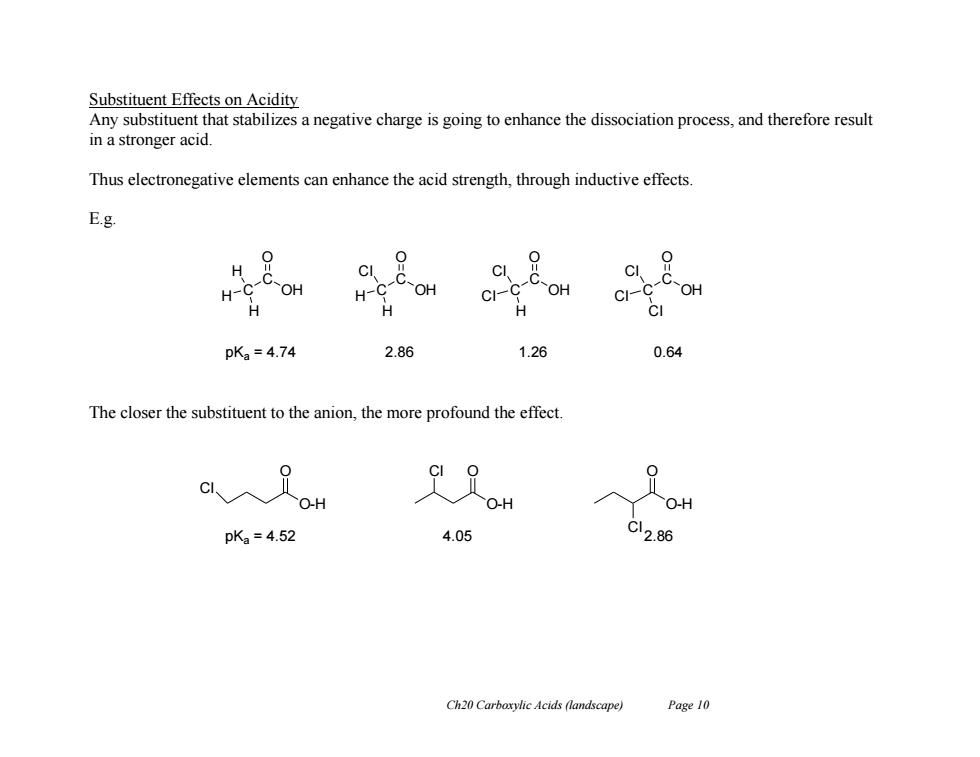
Substituent Effects on Acidity Any substituent that stabilizes a negative charge is going to enhance the dissociation process,and therefore result in a stronger acid. Thus electronegative elements can enhance the acid strength,through inductive effects. E.g. H 9 0 C OH C CI H- H-C OH CI-C OH ci-4 C-OH H H H pKa=4.74 2.86 1.26 0.64 The closer the substituent to the anion,the more profound the effect OH OH O-H pKa=4.52 4.05 C12.86 Ch20 Carboxylic Acids (landscape) Page 10
Ch20 Carboxylic Acids (landscape) Page 10 Substituent Effects on Acidity Any substituent that stabilizes a negative charge is going to enhance the dissociation process, and therefore result in a stronger acid. Thus electronegative elements can enhance the acid strength, through inductive effects. E.g. The closer the substituent to the anion, the more profound the effect. C C OH O H H H C C OH O H Cl H C C OH O Cl Cl H C C OH O Cl Cl Cl pKa = 4.74 2.86 1.26 0.64 O-H O Cl O-H O O-H Cl O Cl pKa = 4.52 4.05 2.86