
Alkenes Properties Nomenclature Stability Addition Reactions
Alkenes Properties Nomenclature Stability Addition Reactions
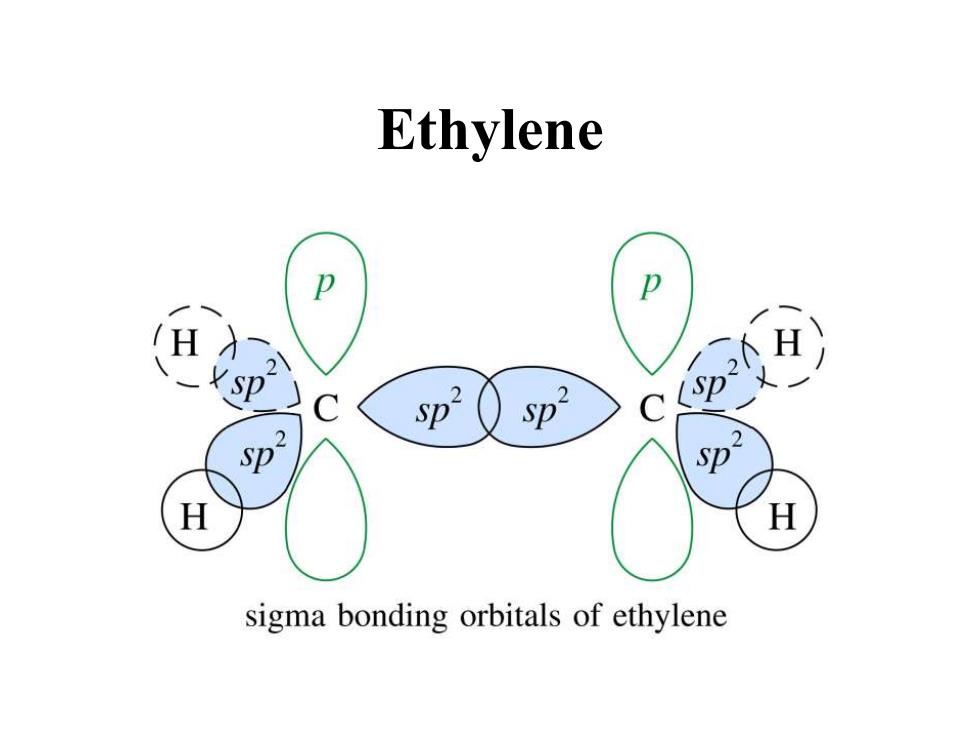
Ethylene p H sp 2 Sp Sp- sp Sp H sigma bonding orbitals of ethylene
Ethylene
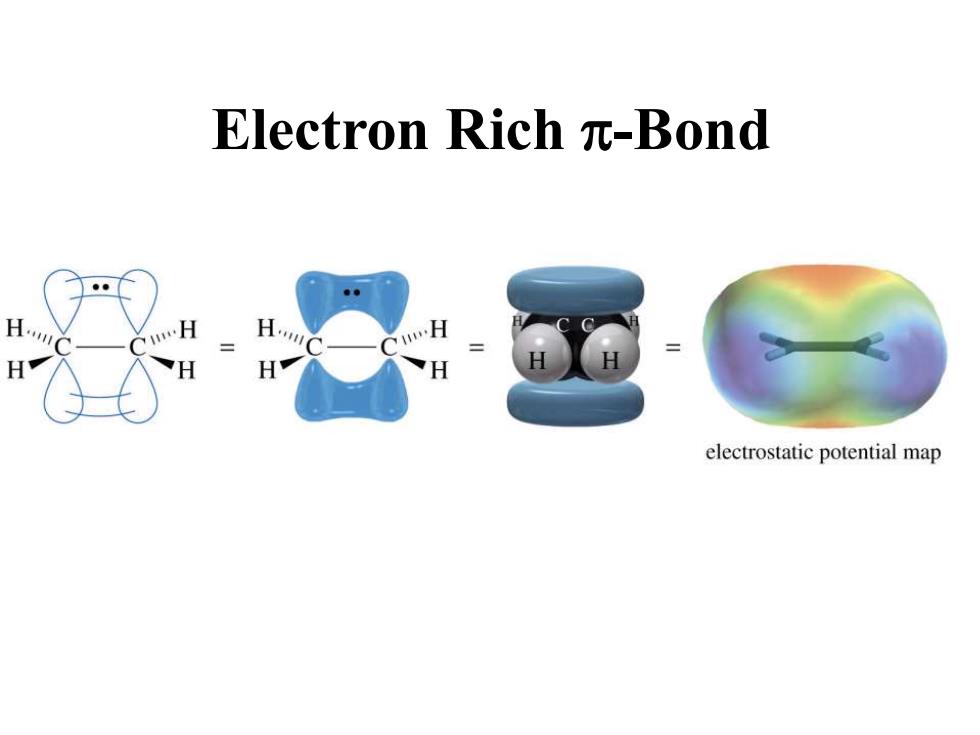
Electron Richπ-Bond .H …H electrostatic potential map
Electron Rich p-Bond
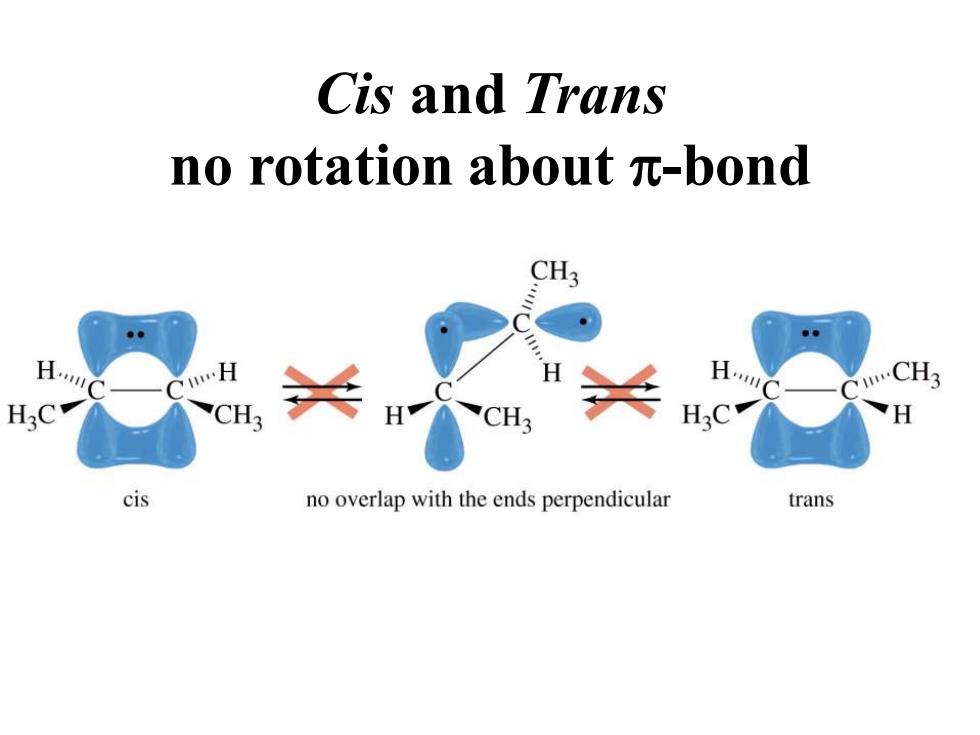
Cis and Trans no rotation about m-bond CH3 CH CH? H:C CH H cis no overlap with the ends perpendicular trans
Cis and Trans no rotation about p-bond
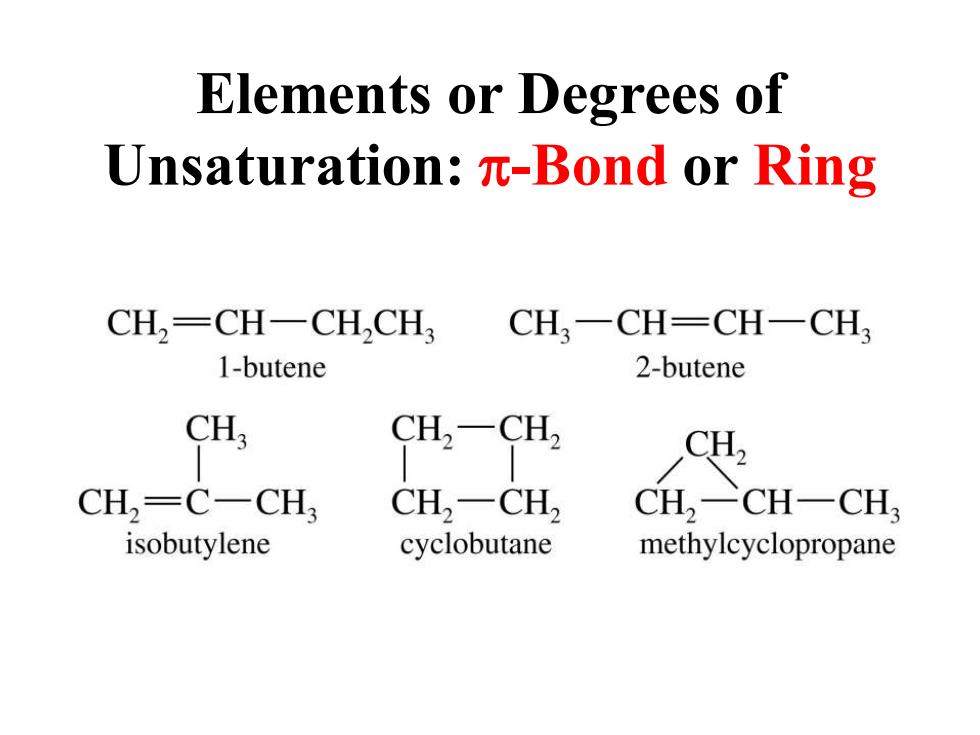
Elements or Degrees of Unsaturation:n-Bond or Ring CH,=CH-CH,CH CH一CH=CH一CH3 1-butene 2-butene CH3 CH-CH, CH2 CH,-C-CH, CH,-CH2 CH,一CH一CH3 isobutylene cyclobutane methylcyclopropane
Elements or Degrees of Unsaturation: p-Bond or Ring
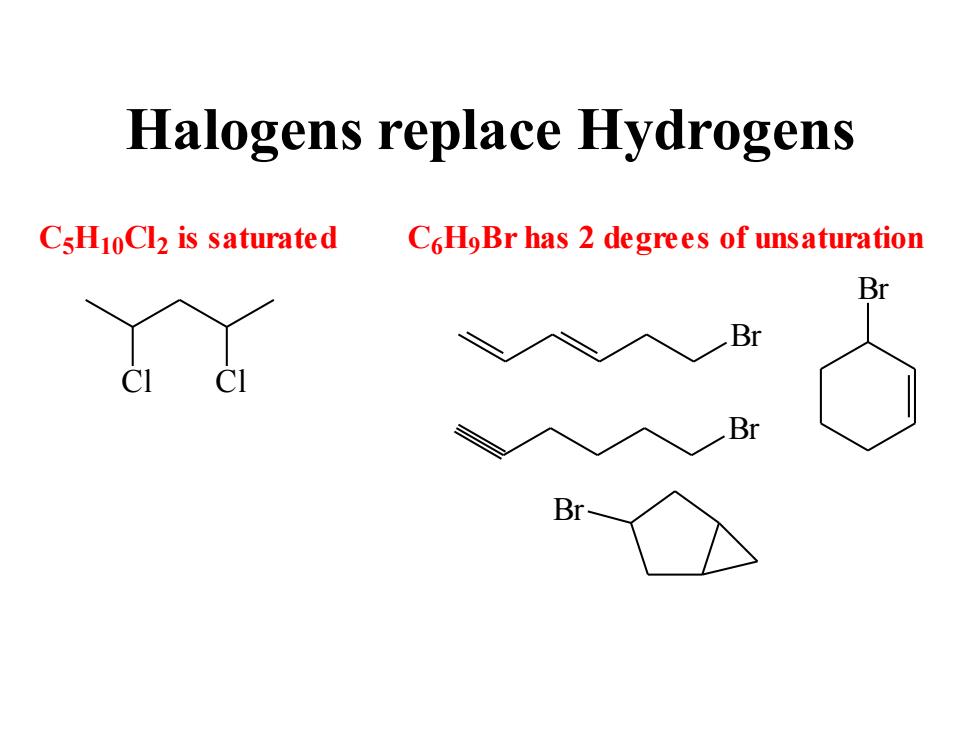
Halogens replace Hydrogens CsH10Cl2 is saturated C6HoBr has 2 degrees of unsaturation Br Br CI B B
Halogens replace Hydrogens C5H10Cl2 is saturated Cl Cl C6H9 Br has 2 degrees of unsaturation Br Br Br Br
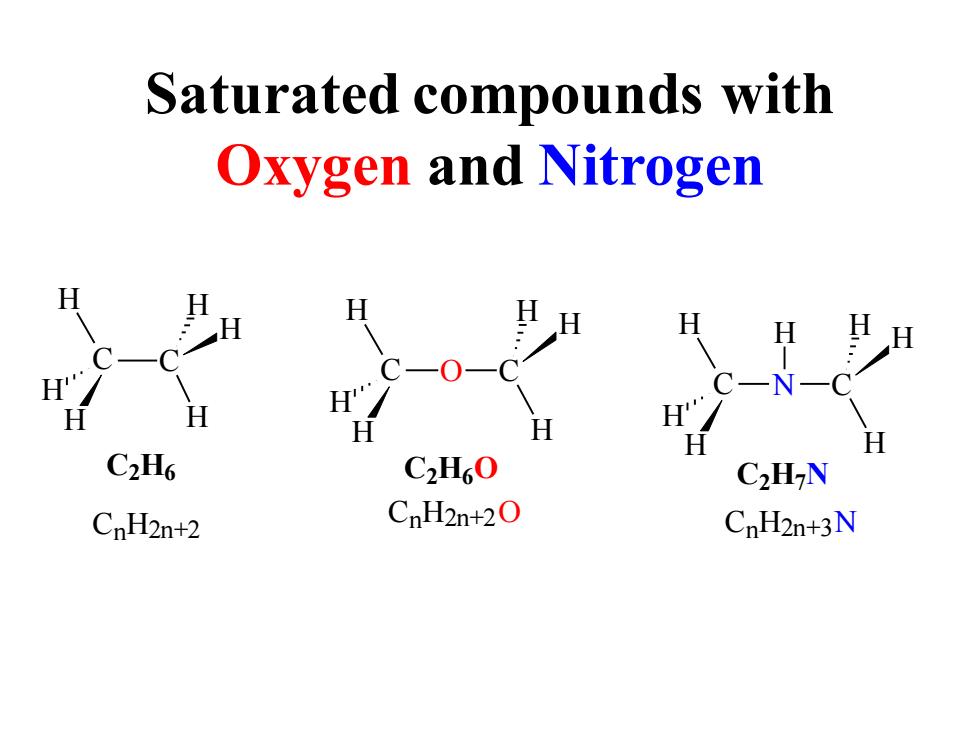
Saturated compounds with Oxygen and Nitrogen H H H H H H H H C2H6 C2H60 C2H-N CnH2n+2 CnH2n+2O CnH2n+3N
Saturated compounds with Oxygen and Nitrogen C C H H H H H H C2H6 H H H C O C H H H C2H6O N C H H H H C H H H C2H7N CnH2n+2 CnH2n+2O CnH2n+3N
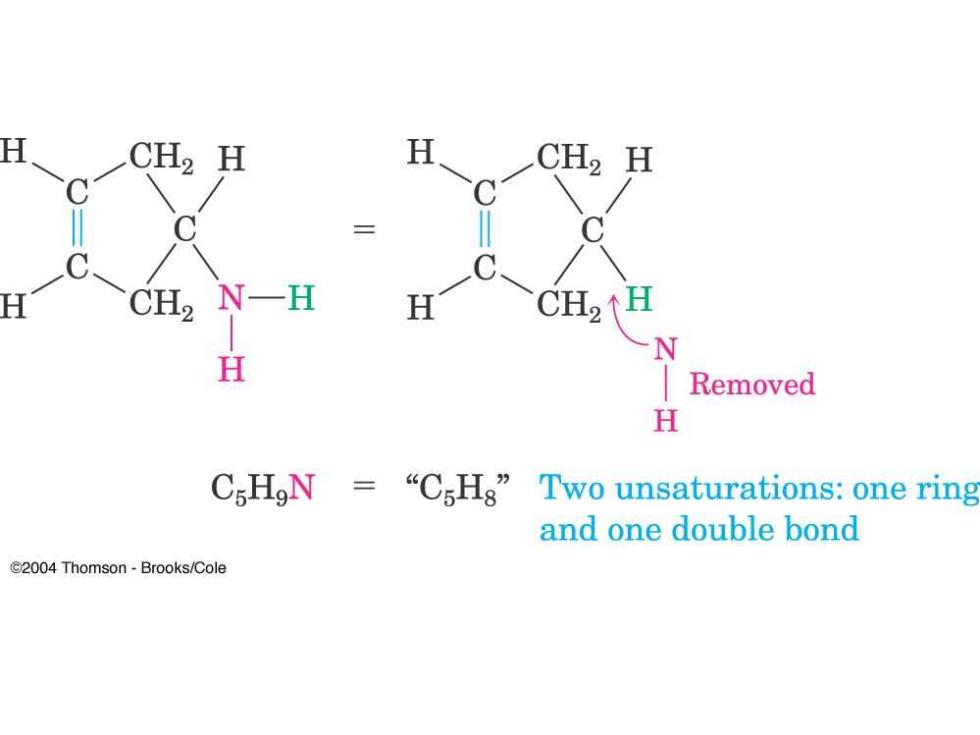
CH2 H H CH2 H CH2 NH H CH2↑H N H Removed H CsHgN=“CsHg”Two unsaturations:one ring and one double bond C2004 Thomson-Brooks/Cole
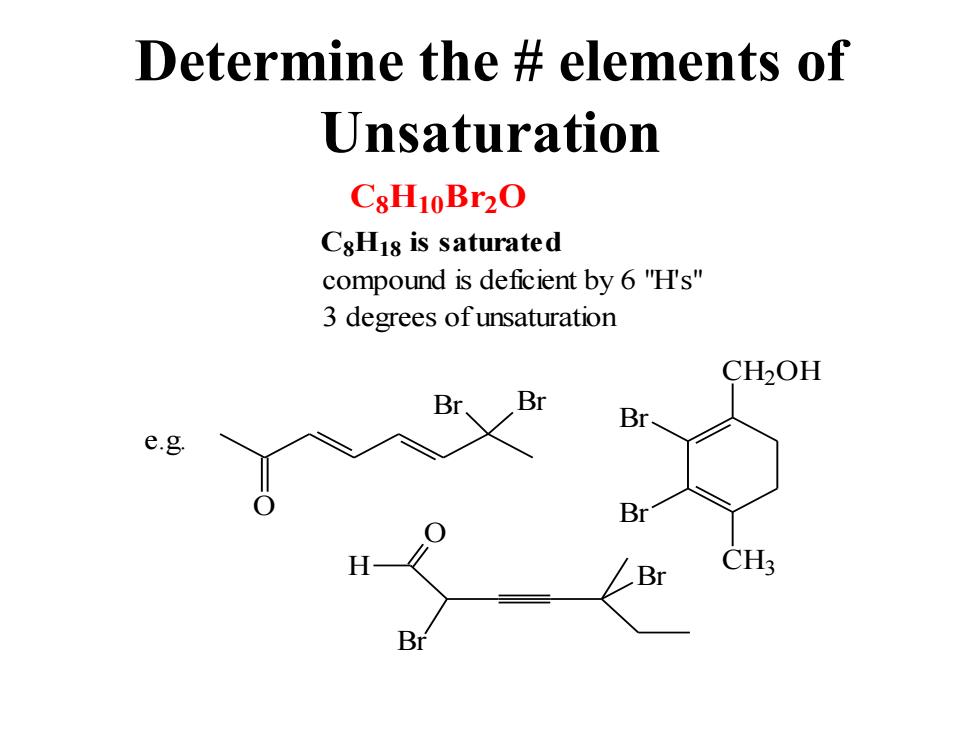
Determine the elements of Unsaturation CsH10Br2O CsHis is saturated compound is deficient by 6"H's" 3 degrees of unsaturation CH2OH Br Br Br e.g Br Br CH3
Determine the # elements of Unsaturation C8H10B r2O C8H18 is saturated compound is deficient by 6 "H's" 3 degrees of unsaturation O Br Br CH2OH CH3 Br Br e.g. O H Br Br
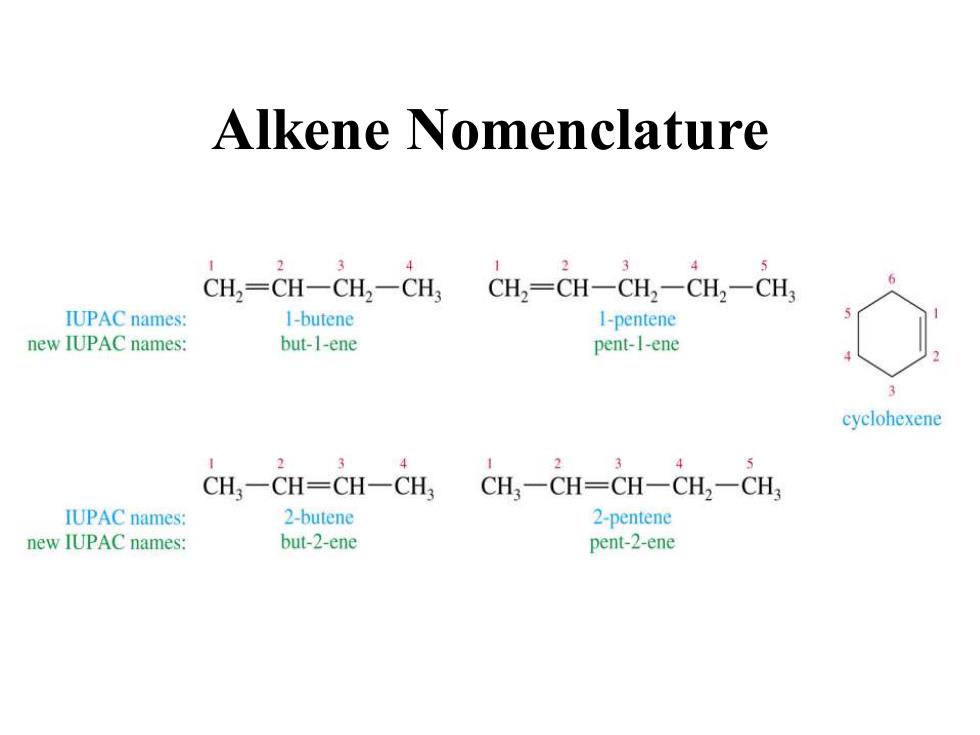
Alkene Nomenclature 4 2 5 CH,=CH一CH2一CH CH2=CH一CH2一CH2一CH IUPAC names: I-butene I-pentene new IUPAC names: but-1-ene pent-1-ene cyclohexene 2 3 4 2 4 CH,一CH=CH一CH CH一CH=CH一CH2一CH IUPAC names: 2-butene 2-pentene new IUPAC names: but-2-ene pent-2-ene
Alkene Nomenclature