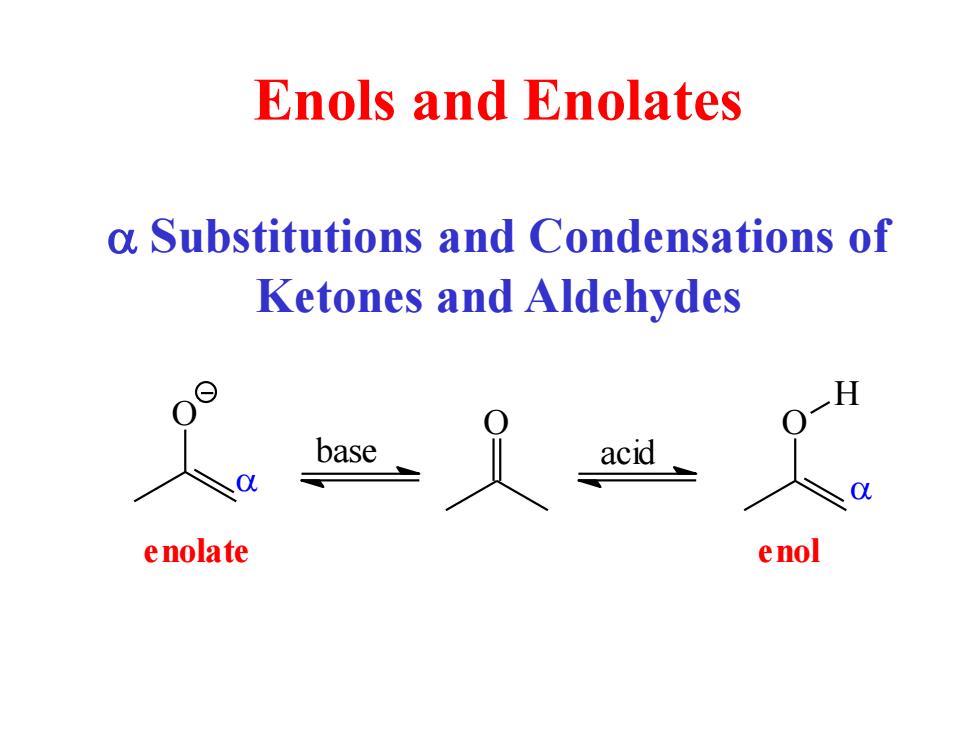
Enols and Enolates a substitutions and Condensations of Ketones and Aldehydes H enolate enol
Enols and Enolates a Substitutions and Condensations of Ketones and Aldehydes O O H O base acid a a enolate enol
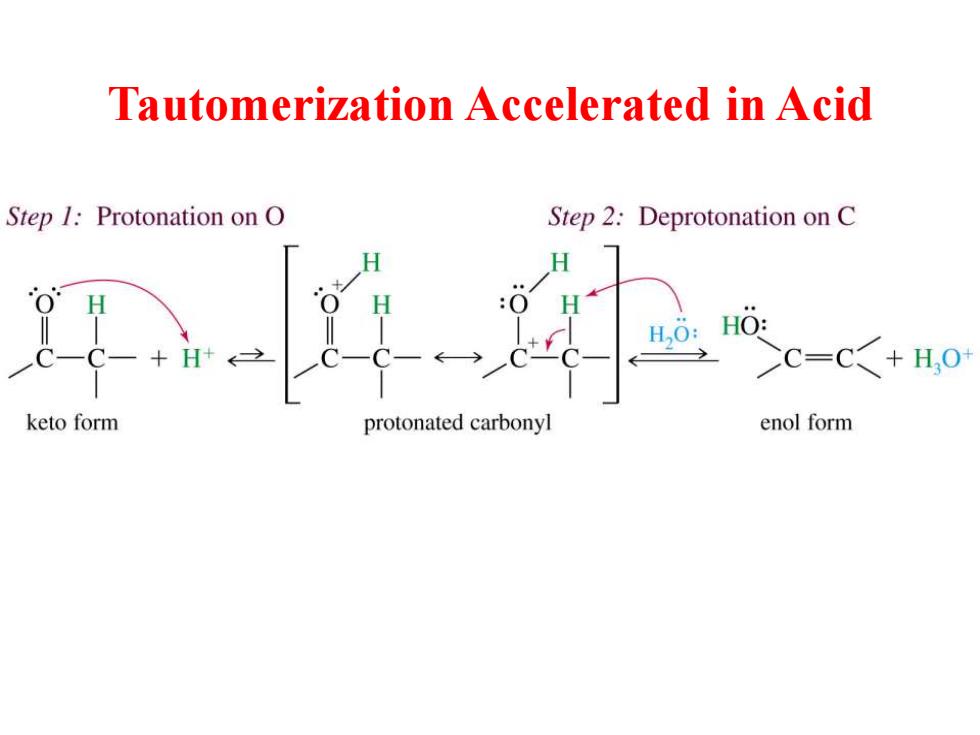
Tautomerization Accelerated in Acid Step 1:Protonation on O Step 2:Deprotonation on C H H H0: HO: C=C<+H,0 keto form protonated carbonyl enol form
Tautomerization Accelerated in Acid
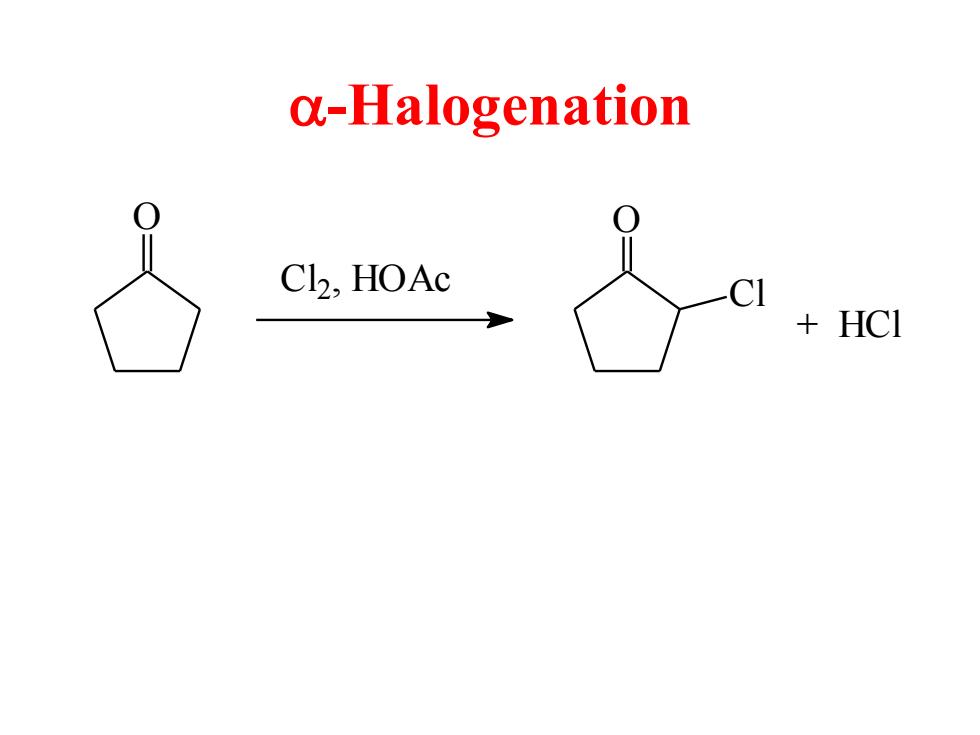
a-Halogenation C12,HOAc CI HCI
a-Halogenation O C l2, HOAc O Cl + HCl
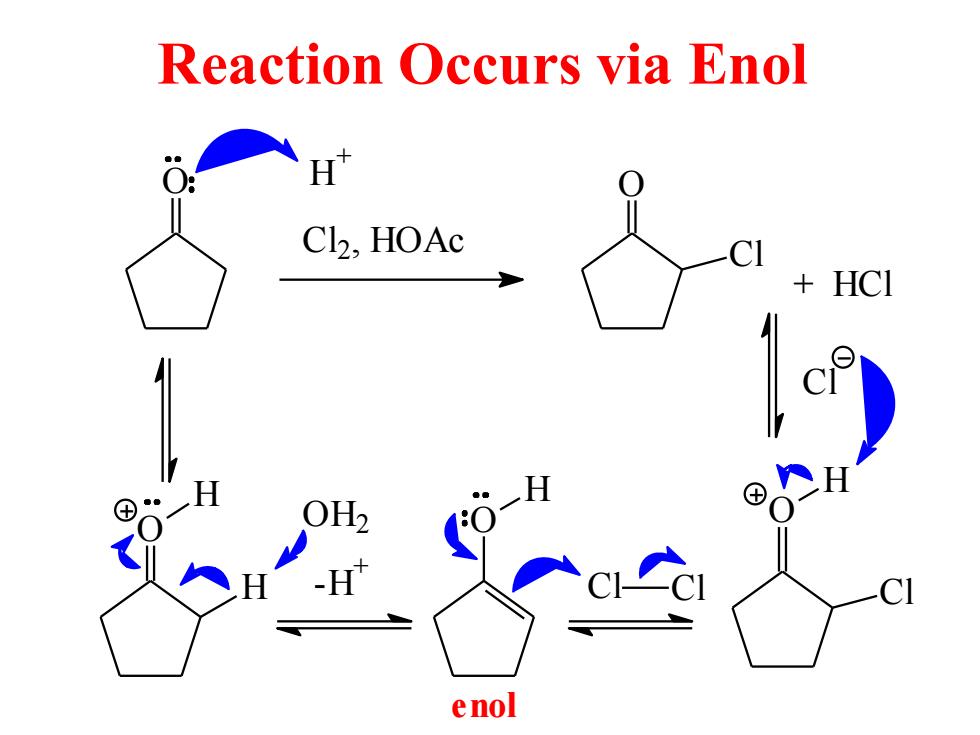
Reaction Occurs via Enol H C12,HOAc HCI enol
Reaction Occurs via Enol O C l2, HOAc O Cl + HCl H + O H H O H - H+ O H2 O H C l Cl Cl C l enol
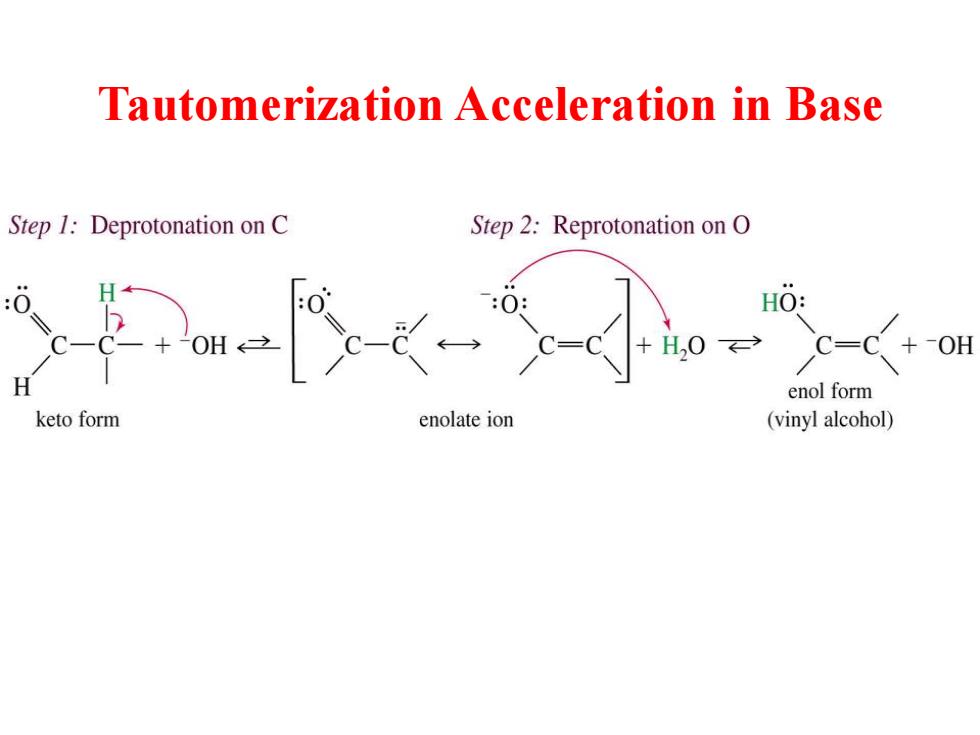
Tautomerization Acceleration in Base Step 1:Deprotonation on C Step 2:Reprotonation on O H0: H0C= -OH enol form keto form enolate ion (vinyl alcohol)
Tautomerization Acceleration in Base

Alkylation in Base Reaction with 1 RX gX- CH2-R+X C-alkylation product (more common) O—CH,一R +X O-alkylation product (less common)
Alkylation in Base Reaction with 1o RX
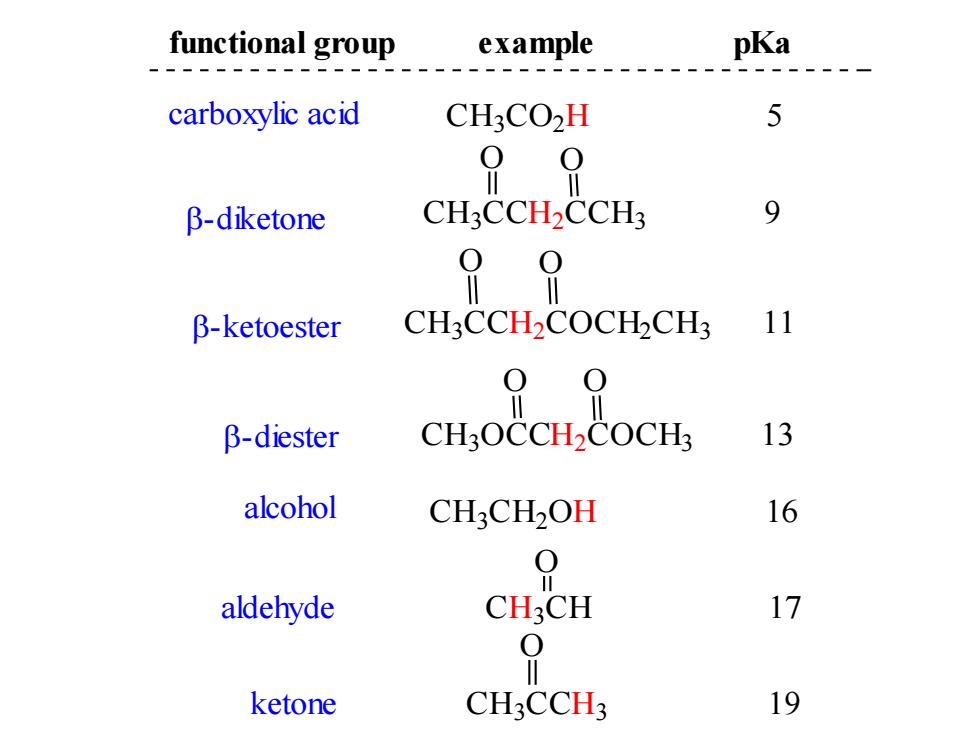
functional group example pKa carboxylic acid CH:CO2H 5 99 B-diketone CH3CCH2CCH3 9 90 B-ketoester CH3CCH2COCHCH? 11 β-diester CH:OCCH2COCH 13 alcohol CH:CH2OH 16 0 aldehyde CH3CH 17 0 ketone CH3CCH3 19
C H3 C O2 H 5 C H3 C CH2 CCH3 9 O O pKa C H3 C CH2 COCH2 C H3 11 O O C H3 OCCH2 COCH3 13 O O C H3 C H2 OH 16 carboxylic acid -diketone -ketoester -diester alcohol functional group example aldehyde CH3 CH 17 O ketone CH3 C CH3 19 O
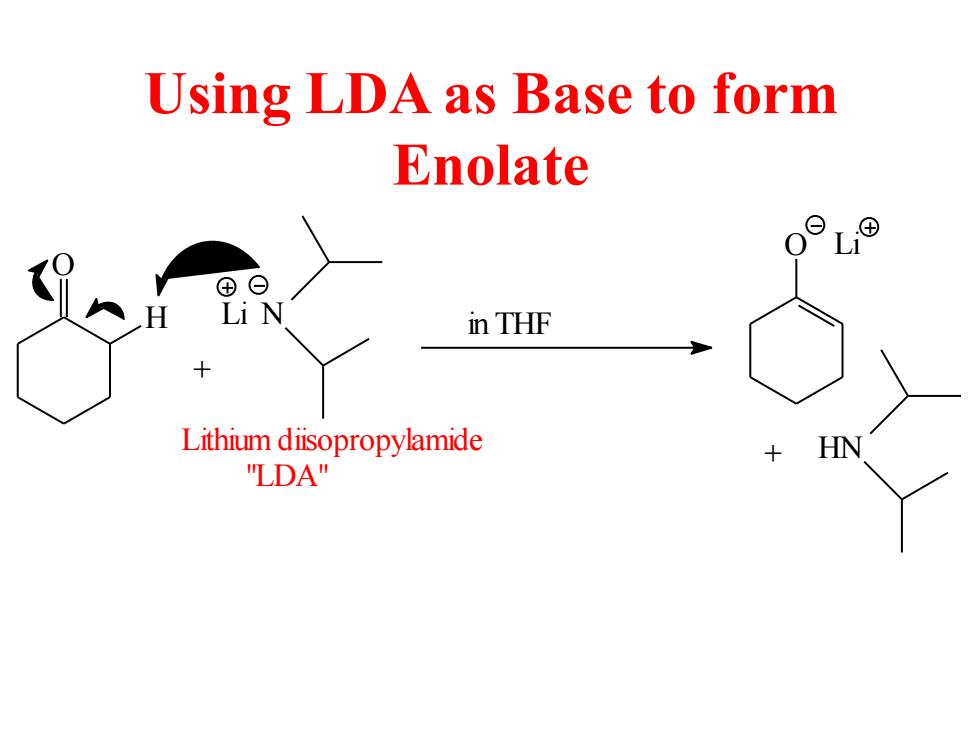
Using LDA as Base to form Enolate ⊕日 in THF Lithium diisopropylamide HN "LDA
Using LDA as Base to form Enolate O H Li N Lithium diisopropylamide + O Li + HN "LDA" in THF
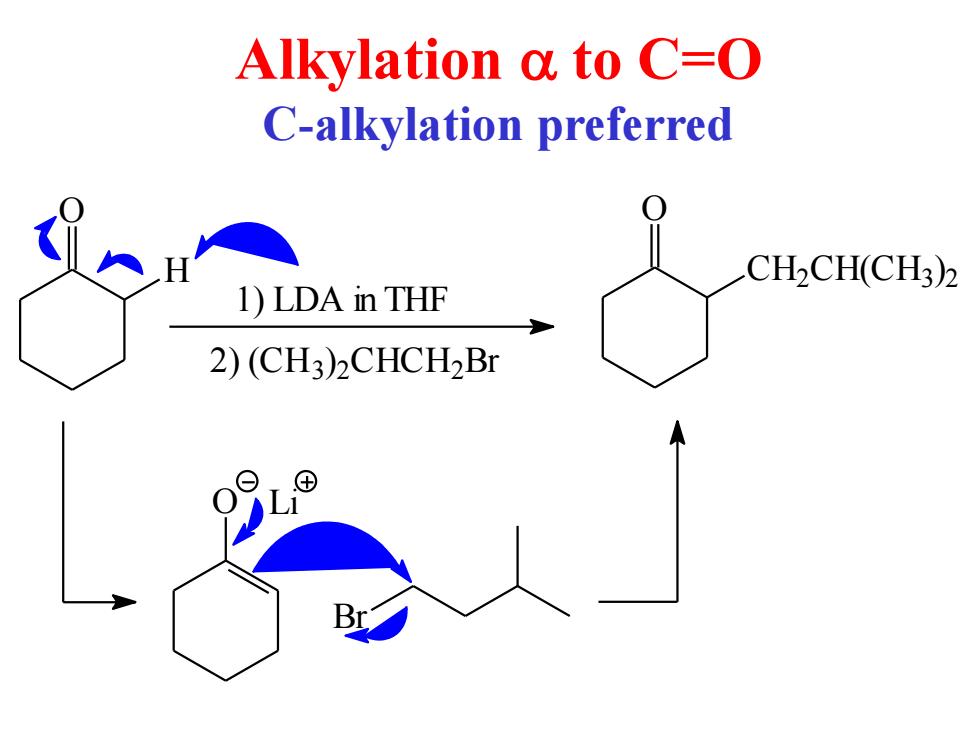
Alkylation a to C=O C-alkylation preferred CH2CH(CH3)2 1)LDA in THF 2)(CH3)CHCH2Br ⊕
Alkylation a to C=O C-alkylation preferred O H 1) LDA in THF 2) (CH3) 2 CHCH2 Br O CH2 CH(CH3) 2 O Li Br
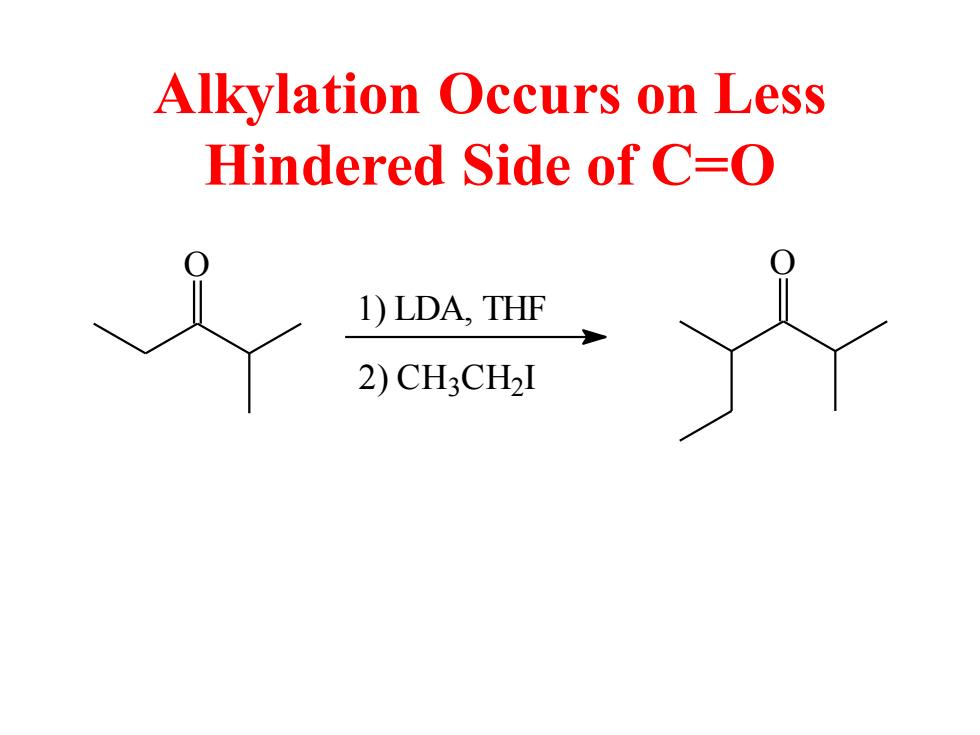
Alkylation Occurs on Less Hindered Side of C=O 1)LDA,THF 2)CH3CH2I
Alkylation Occurs on Less Hindered Side of C=O O 1) LDA, THF 2) CH3 C H2 I O