Ketones and Aldehydes Properties Nomenclature Preparation Reactions Synthesis
Ketones and Aldehydes Properties Nomenclature Preparation Reactions Synthesis
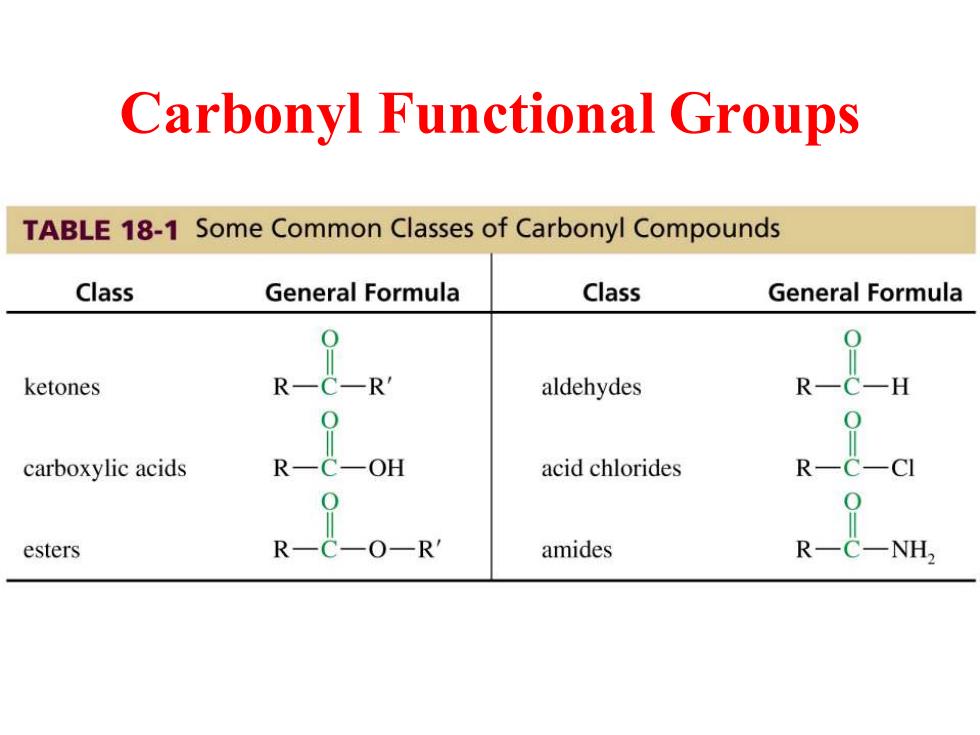
Carbonyl Functional Groups TABLE 18-1 Some Common Classes of Carbonyl Compounds Class General Formula Class General Formula ketones R aldehydes R -H carboxylic acids R acid chlorides R— esters P R amides
Carbonyl Functional Groups
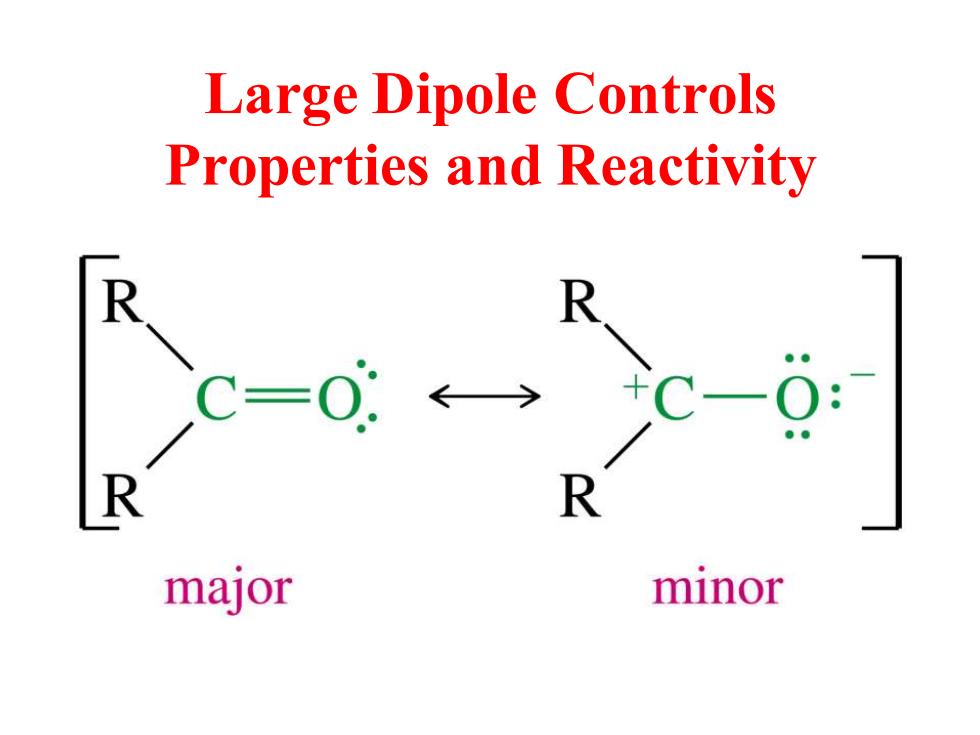
Large Dipole Controls Properties and Reactivity R R major minor
Large Dipole Controls Properties and Reactivity
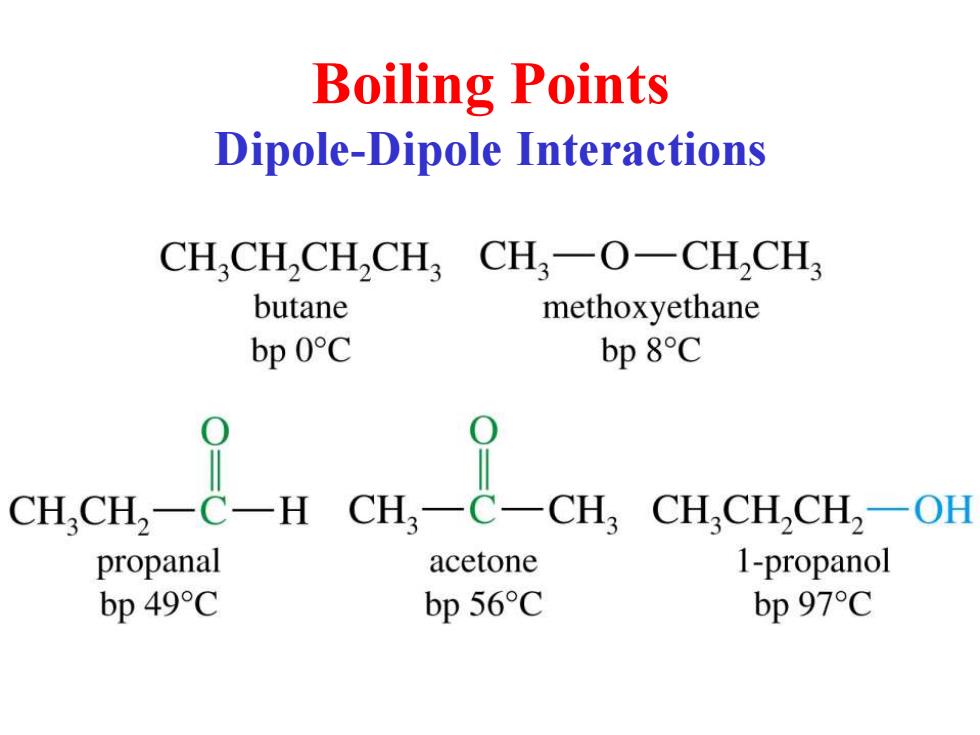
Boiling Points Dipole-Dipole Interactions CHCHCH,CH,CH-O-CH,CH, butane methoxyethane bp 0C bp 8C 0 CH;CH,-C-H CH,-C-CH( CHCH,CH2一OH propanal acetone 1-propanol bp 49C bp56°C bp 97C
Boiling Points Dipole-Dipole Interactions
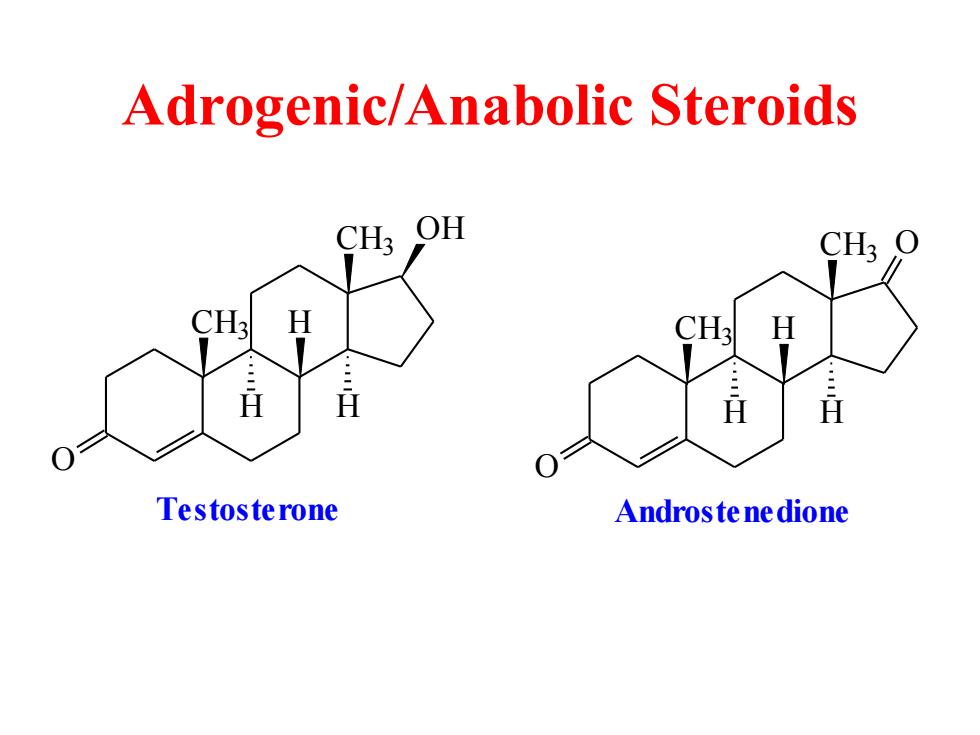
Adrogenic/Anabolic Steroids CH3 OH H H Testosterone Androstenedione
Adrogenic/Anabolic Steroids CH3 O CH3 OH H H H Testosterone CH3 O CH3 H H H O Androstenedione
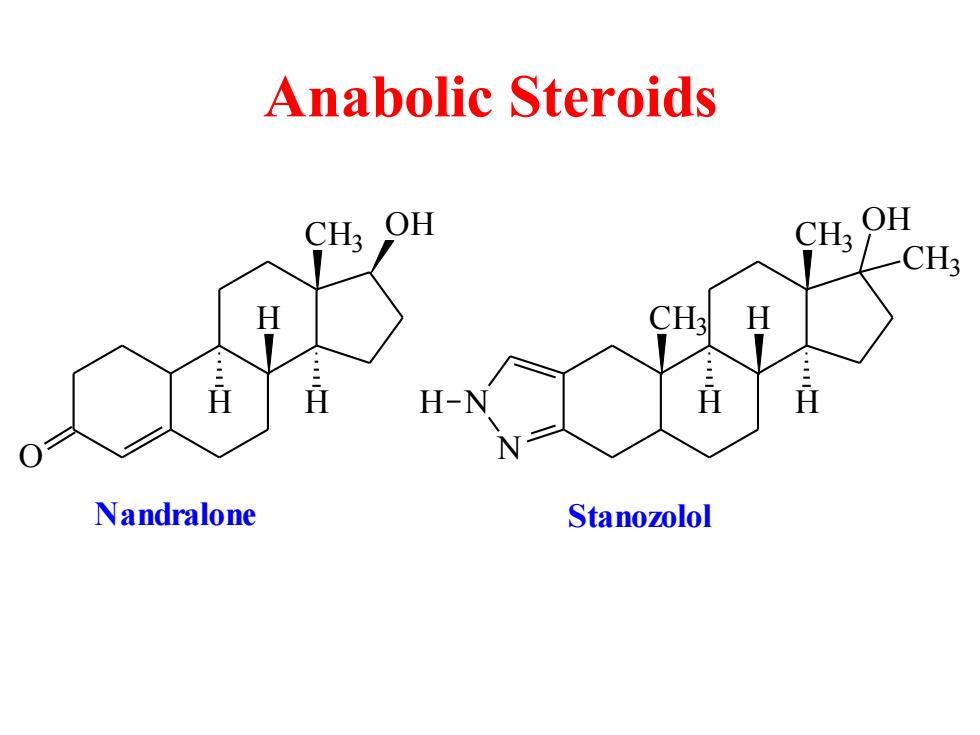
Anabolic Steroids CH3 OH OH CH3 H H-N H H Nandralone Stanozolol
Anabolic Steroids O CH3 OH H H H CH3 CH3 H H H N N CH3 OH H Nandralone Stanozolol
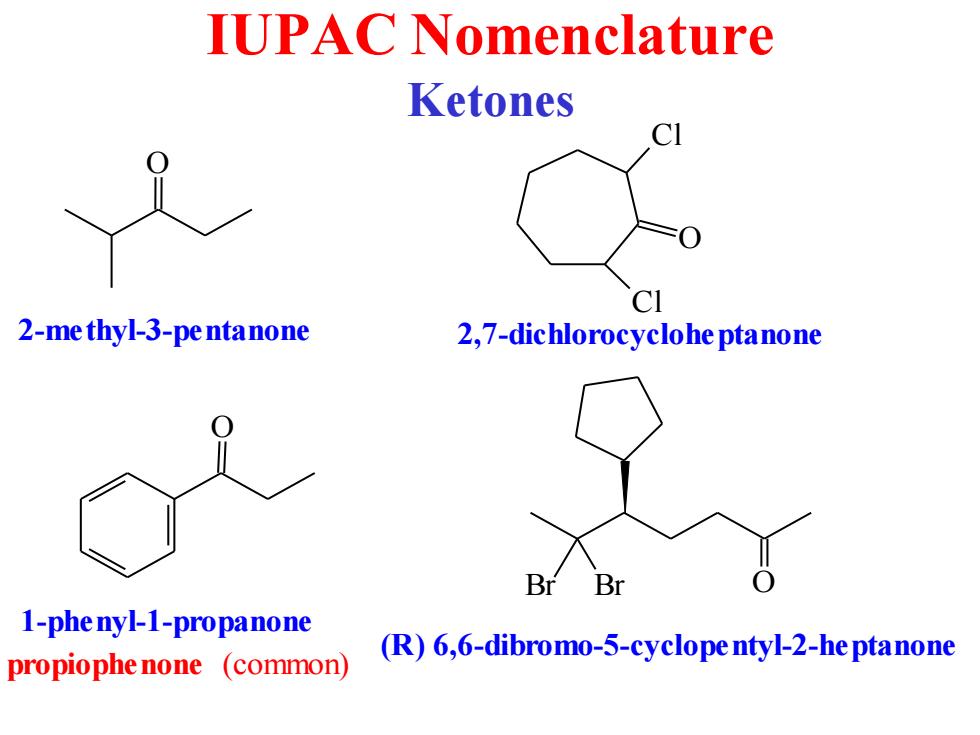
IUPAC Nomenclature Ketones CI 2-me thyl-3-pe ntanone 2,7-dichlorocyclohe ptanone Br Br 1-phe nyl-1-propanone (R)6,6-dibromo-5-cyclope ntyl-2-he ptanone propiophe none (common)
IUPAC Nomenclature Ketones O 2-methyl-3-pentanone O Cl Cl 2,7-dichlorocycloheptanone O 1-phenyl-1-propanone propiophenone (common) Br Br O (R) 6,6-dibromo-5-cyclopentyl-2-heptanone
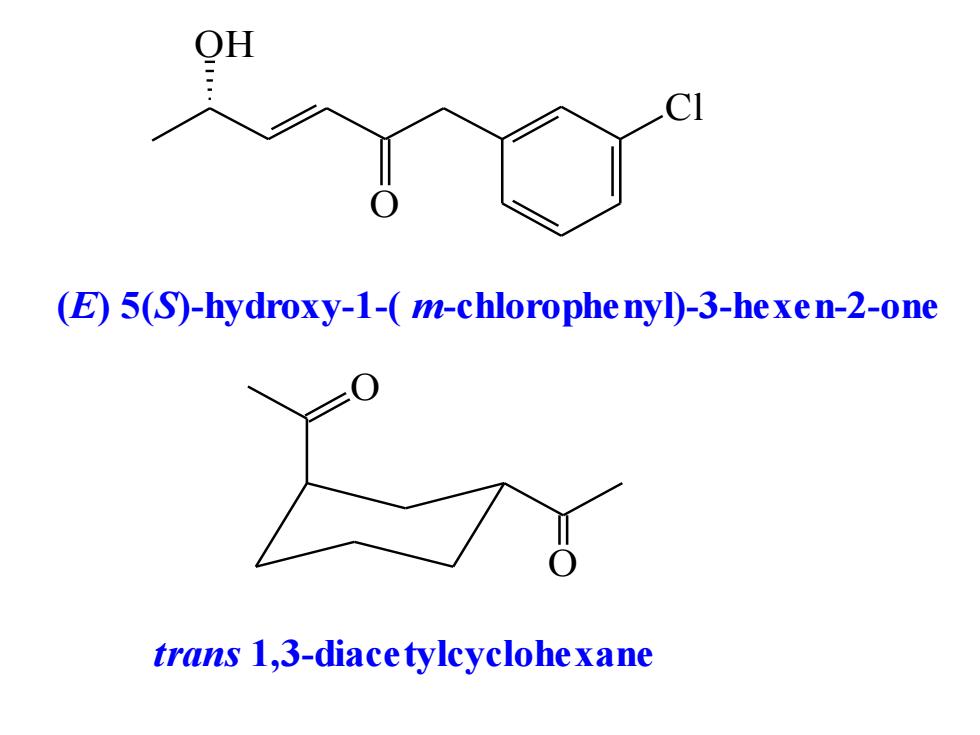
OH (E)5(S)-hydroxy-1-(m-chlorophe nyl)-3-hexen-2-one trans 1,3-diacetylcyclohexane
OH O Cl (E) 5(S)-hydroxy-1-( m-chlorophenyl)-3-hexen-2-one O O trans 1,3-diacetylcyclohexane
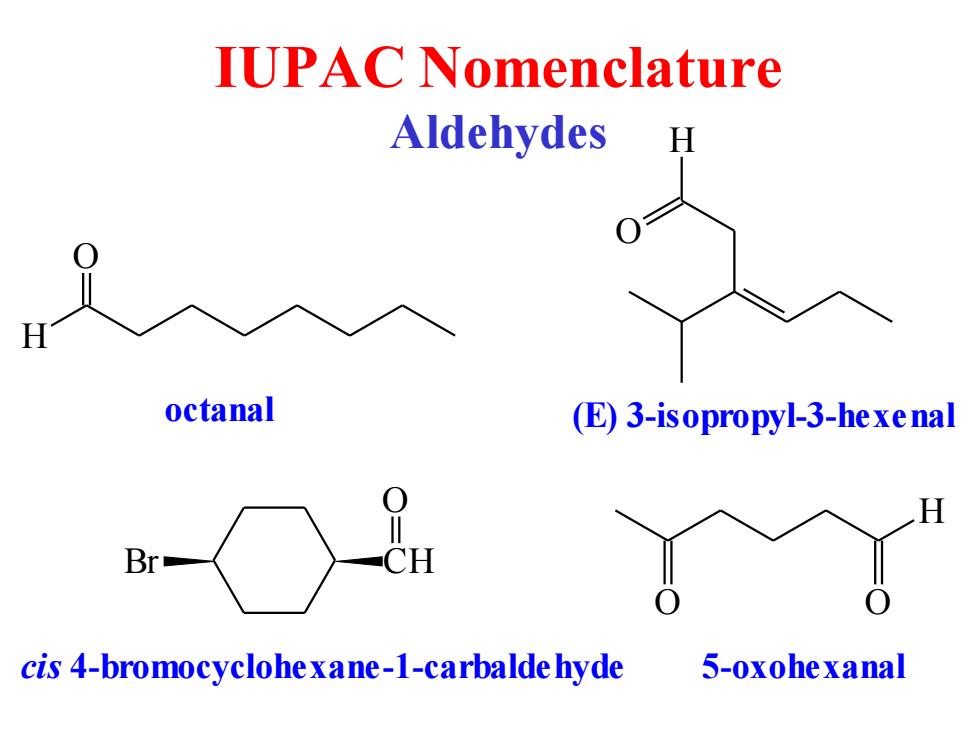
IUPAC Nomenclature Aldehydes H octanal (E)3-isopropyl-3-hexenal H Br人 CH 0 cis 4-bromocyclohe xane-1-carbalde hyde 5-oxohexanal
IUPAC Nomenclature Aldehydes O H octanal H O (E) 3-isopropyl-3-hexenal CH O Br cis 4-bromocyclohexane-1-carbaldehyde O H O 5-oxohexanal
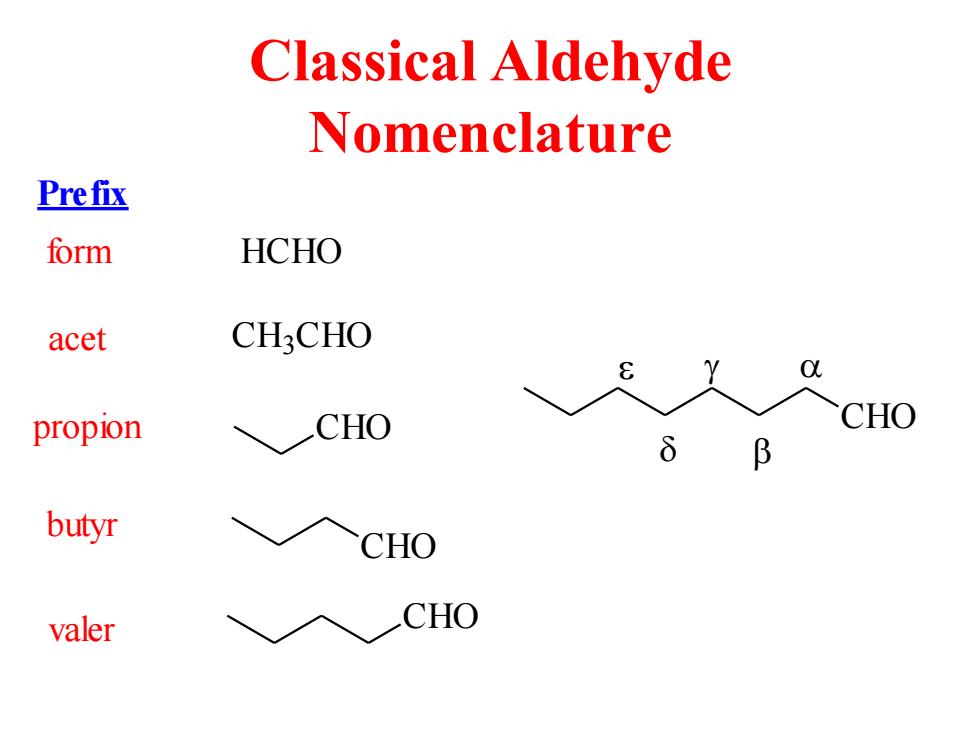
Classical Aldehyde Nomenclature Prefix form HCHO acet CH3CHO propion CHO CHO B butyr CHO valer CHO
Classical Aldehyde Nomenclature HCHO C H3 CHO CHO CHO CHO Prefix form acet propion butyr valer CHO