
Structure and Bonding of Organic Molecules Orbital Theory Hybridization and Geometry Polarity Functional Groups
Structure and Bonding of Organic Molecules Orbital Theory Hybridization and Geometry Polarity Functional Groups
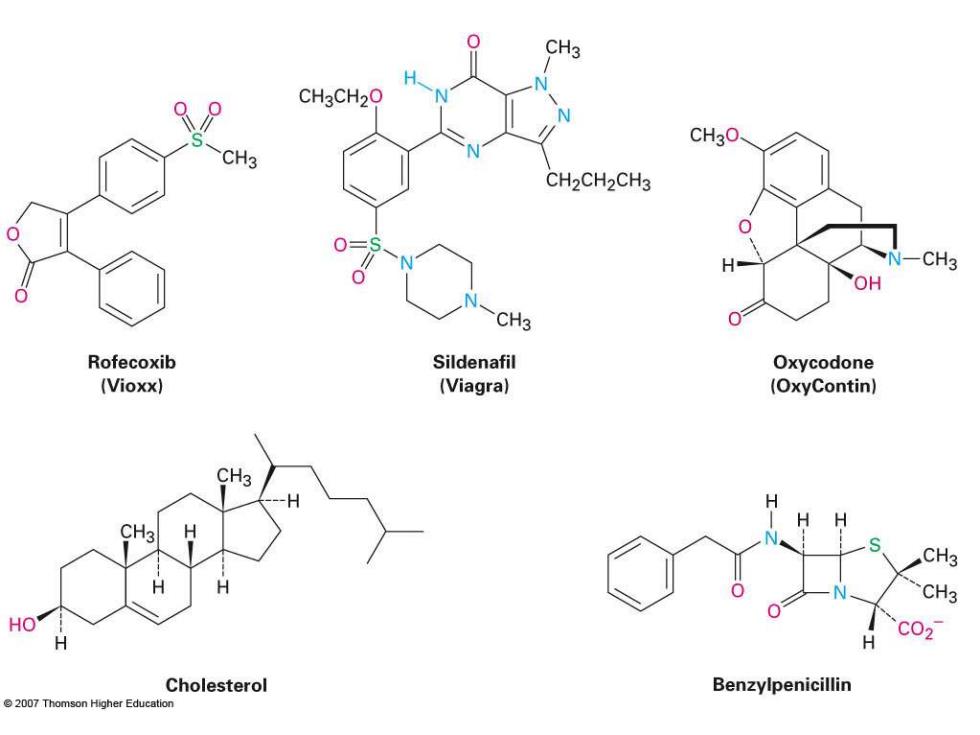
CH3 CH3CH20 CH3O CH3 CH2CH2CH3 CH3 OH 0 CH3 Rofecoxib Sildenafil Oxycodone (Vioxx) (Viagra) (OxyContin) CH3 H S CH3 CH3 HO H H C02 Cholesterol Benzylpenicillin 2007 Thomson Higher Education
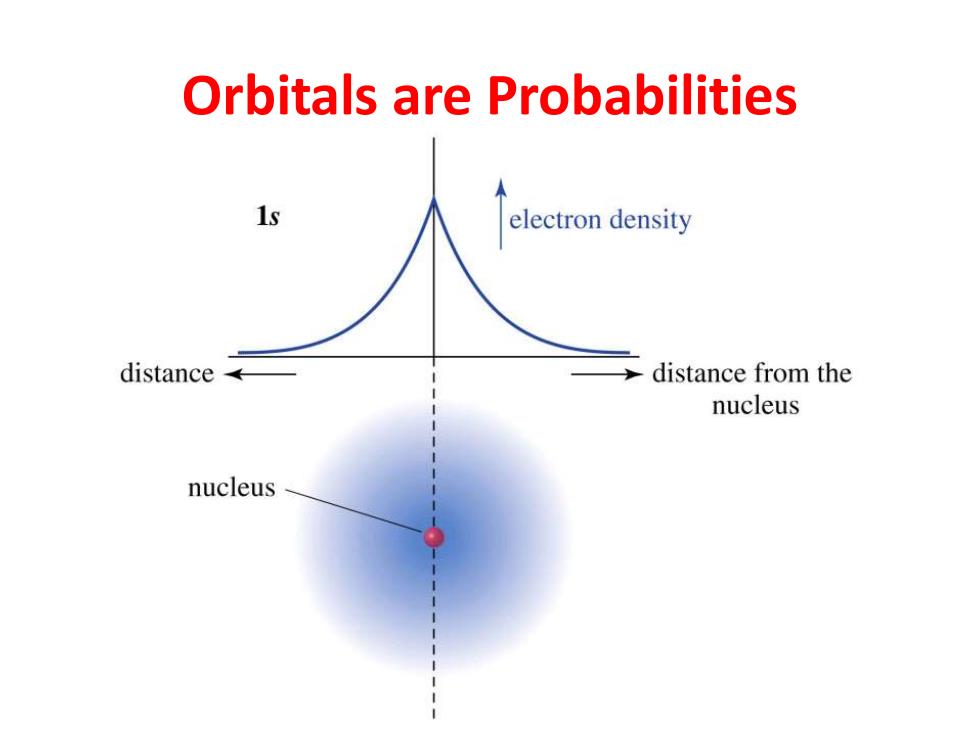
Orbitals are Probabilities 1s electron density distance distance from the nucleus nucleus
Orbitals are Probabilities
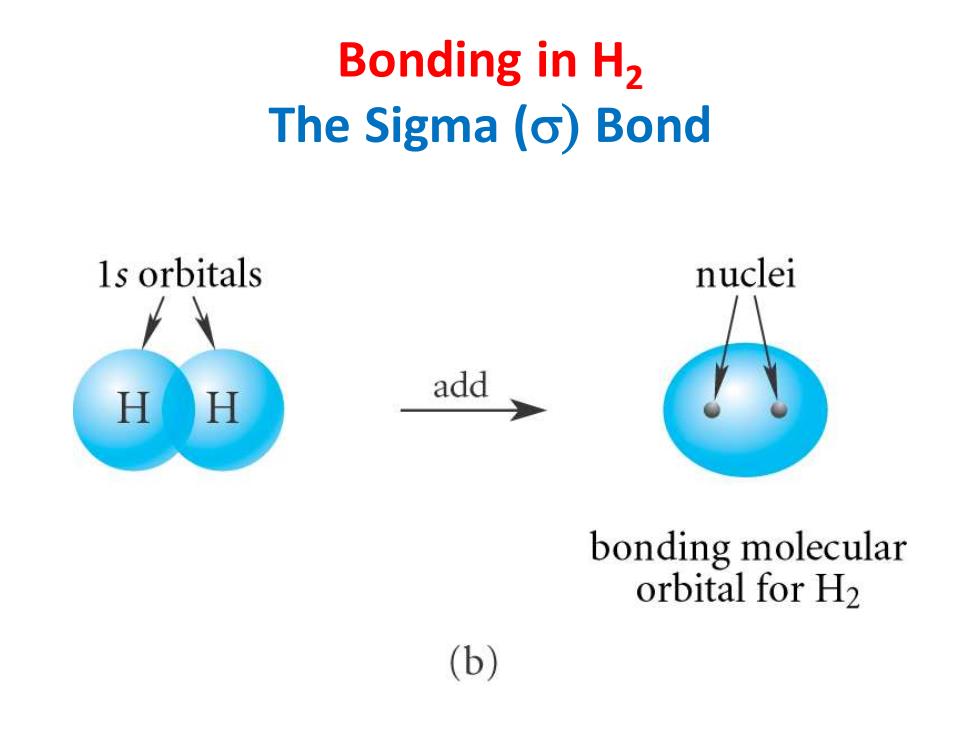
Bonding in H2 The Sigma (o)Bond 1s orbitals nuclei add bonding molecular orbital for H2 (b)
Bonding in H2 The Sigma (s) Bond
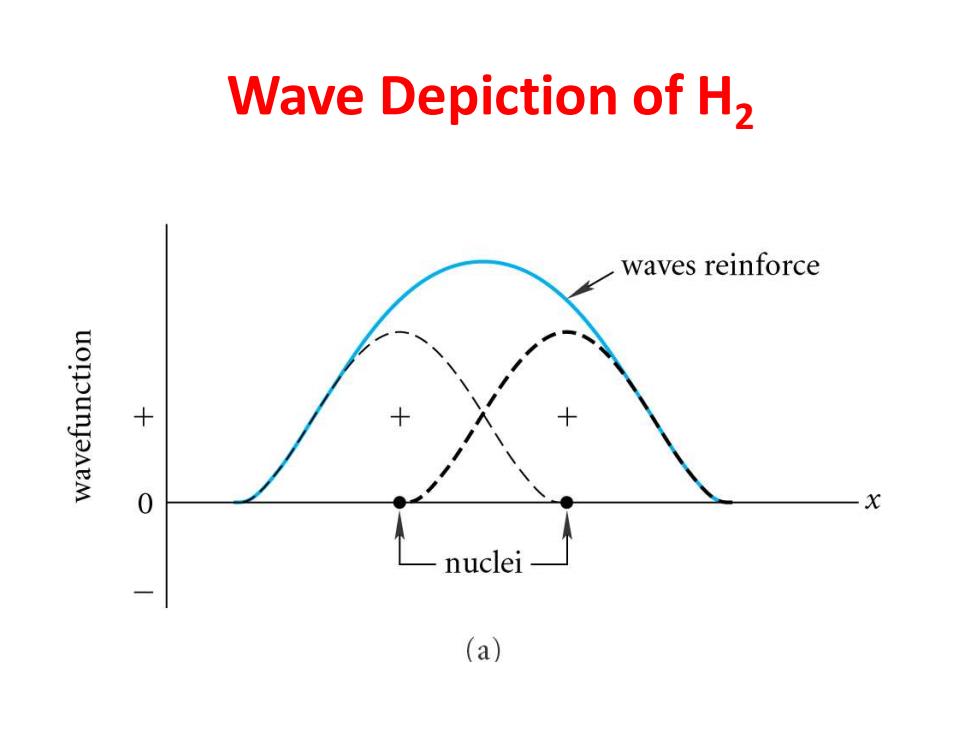
Wave Depiction of H2 waves reinforce uonounJaneM 0 X nuclei (a)
Wave Depiction of H2
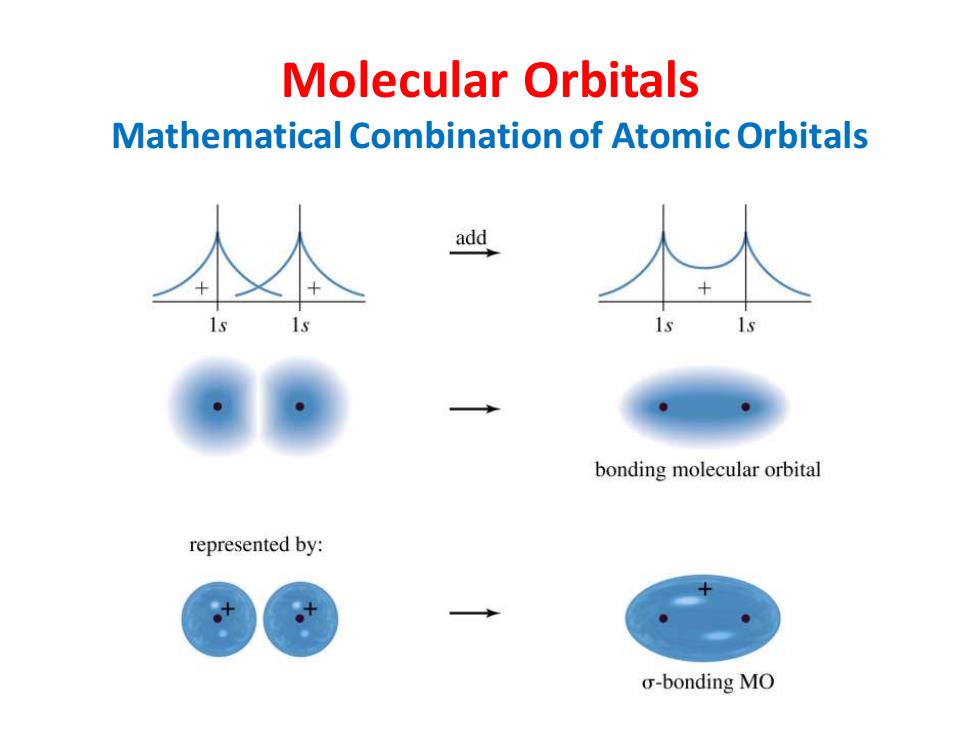
Molecular Orbitals Mathematical Combination of Atomic Orbitals add bonding molecular orbital represented by: o-bonding MO
Molecular Orbitals Mathematical Combination of Atomic Orbitals
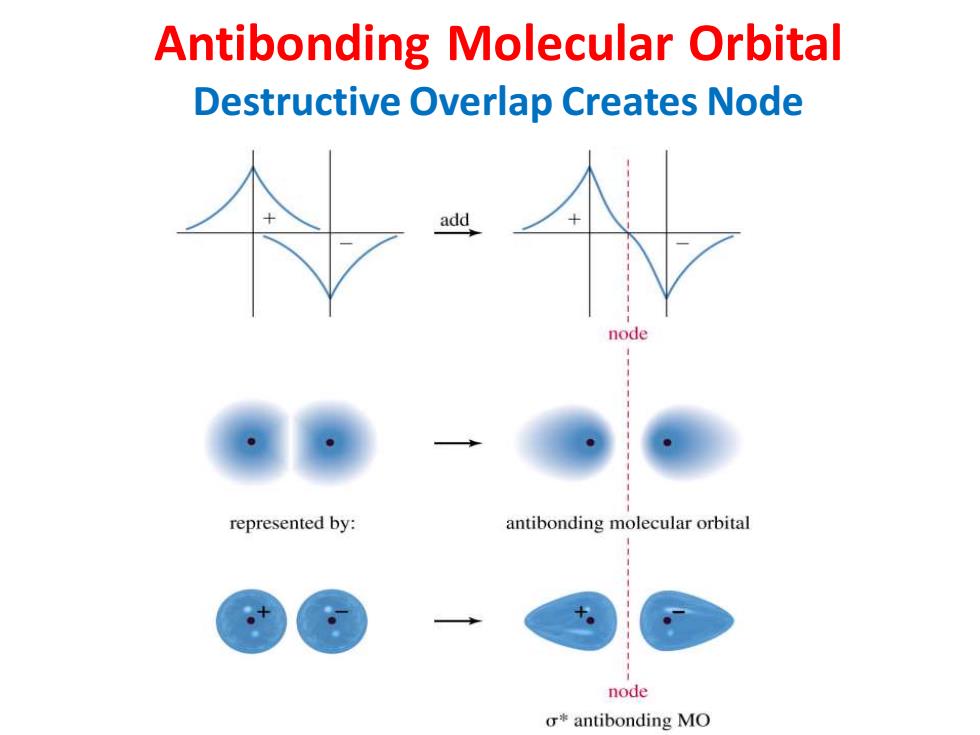
Antibonding Molecular Orbital Destructive Overlap Creates Node add node represented by: antibonding molecular orbital node σantibonding MO
Antibonding Molecular Orbital Destructive Overlap Creates Node
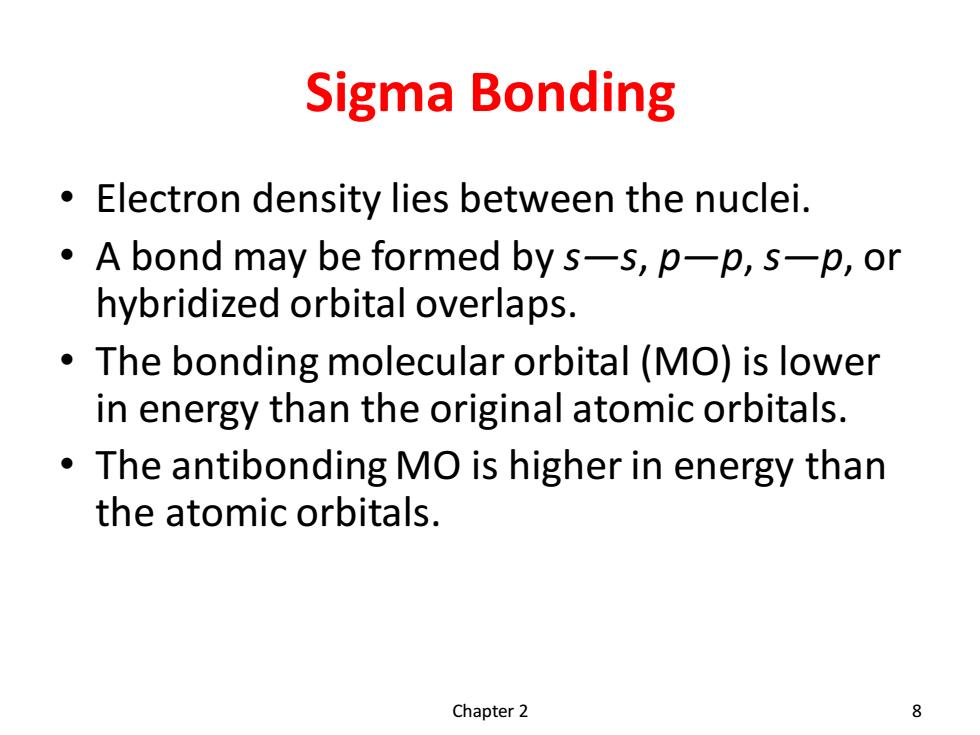
Sigma Bonding Electron density lies between the nuclei. A bond may be formed by s-s,p-p,s-p,or hybridized orbital overlaps. The bonding molecular orbital (MO)is lower in energy than the original atomic orbitals. The antibonding MO is higher in energy than the atomic orbitals. Chapter 2 8
Sigma Bonding • Electron density lies between the nuclei. • A bond may be formed by s—s, p—p, s—p, or hybridized orbital overlaps. • The bonding molecular orbital (MO) is lower in energy than the original atomic orbitals. • The antibonding MO is higher in energy than the atomic orbitals. Chapter 2 8
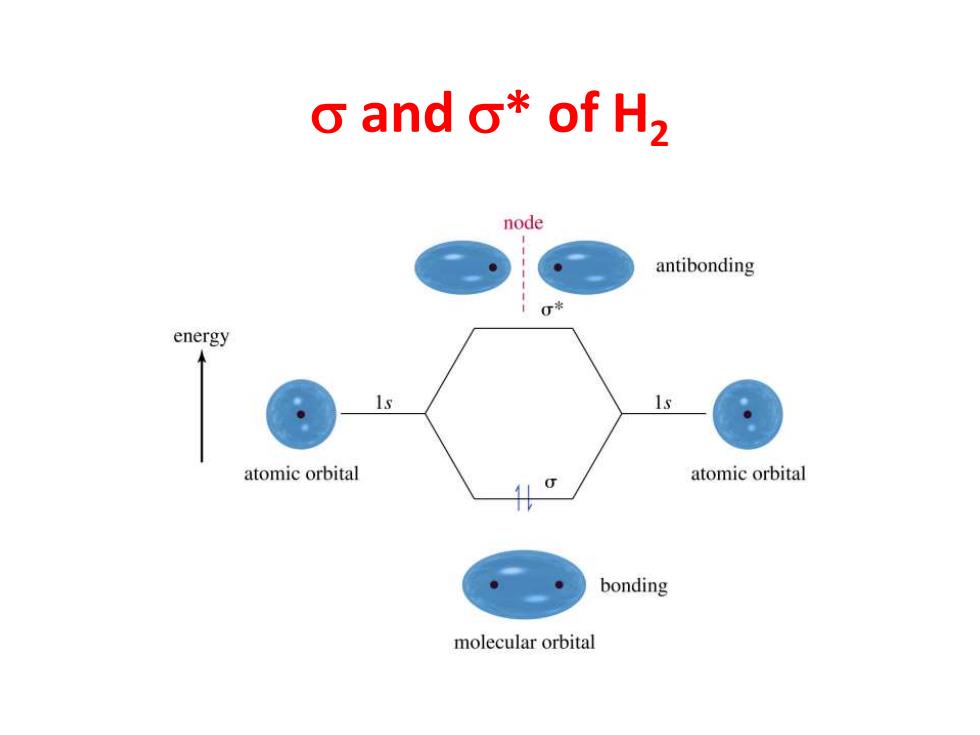
o and o*of H2 node antibonding energy atomic orbital 0 atomic orbital bonding molecular orbital
s and s* of H2
Electron Configurations d 3s 2p 2s 1s
Electron Configurations