Structure and Synthesis of Alcohols Biological Activity Nomenclature Preparation Reactions
Structure and Synthesis of Alcohols Biological Activity Nomenclature Preparation Reactions

Structure of Water and Methanol 0.96A 1.4A 0.96 日104.5 108.9 H water water water methyl alcohol methyl alcohol methyl alcohol o2013 Poarson Educe谢eni的 Oxygen is sp3 hybridized and tetrahedral. ·TheH-O-H angle in water is 104.5° ·TheC-O-H angle in methyl alcohol is 108.9°. 2013 Pearson Education,Inc Chapter 10 2
© 2013 Pearson Education, Inc. Chapter 10 2 Structure of Water and Methanol • Oxygen is sp3 hybridized and tetrahedral. • The H—O—H angle in water is 104.5°. • The C—O—H angle in methyl alcohol is 108.9°
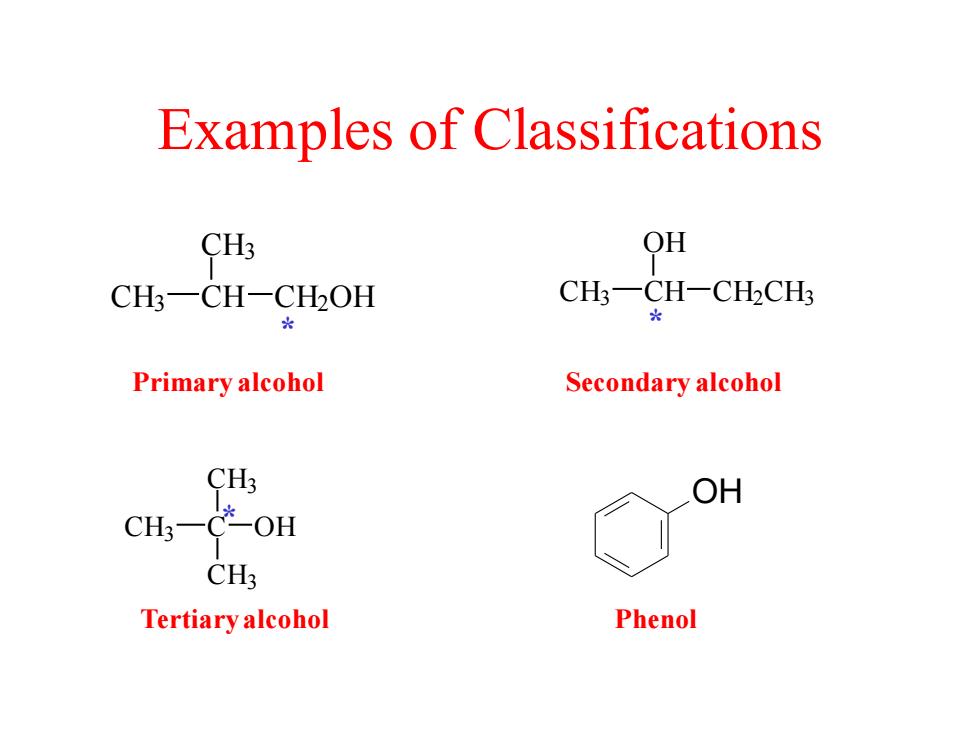
Examples of Classifications CH3 QH CH;-CH-CH2OH CH一CH-CH2CH Primary alcohol Secondary alcohol CH3 OH CH3一 OH CH3 Tertiary alcohol Phenol
Examples of Classifications CH3 C CH3 CH3 * OH CH3 CH OH CH2CH3 * CH3 CH CH3 CH2OH * Primary alcohol Secondary alcohol OH Tertiary alcohol Phenol
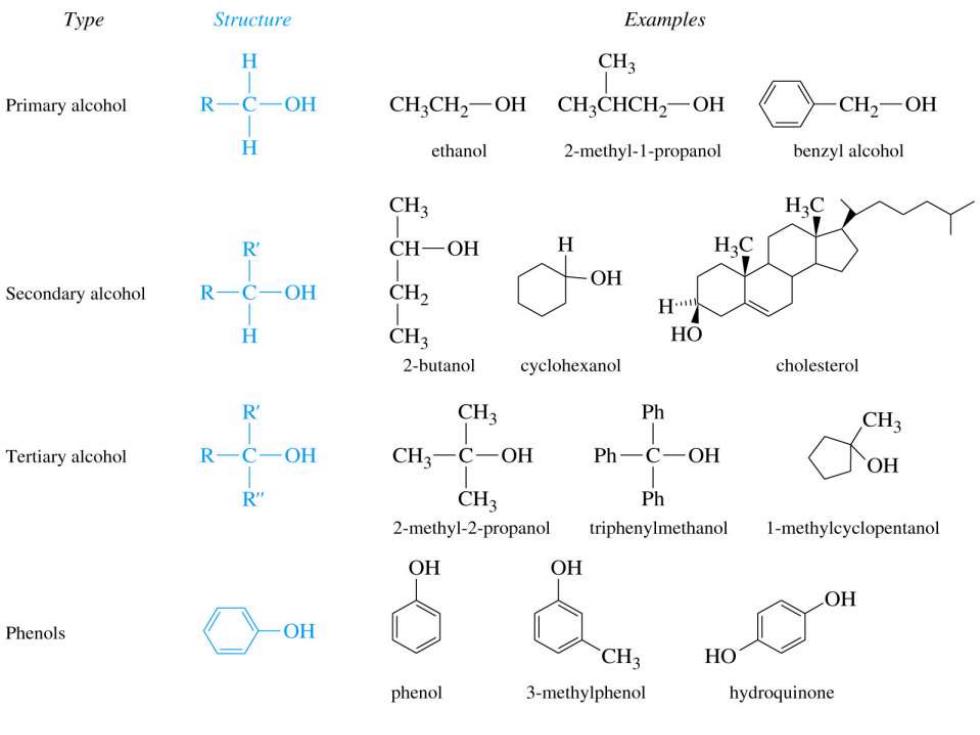
Type Structure Examples H CH3 Primary alcohol OH CH3CH2-OH CH3CHCH2一OH CH2一OH ethanol 2-methyl-1-propanol benzyl alcohol CH3 R CH-OH H OH Secondary alcohol OH CH2 H CH3 HO 2-butanol cyclohexanol cholesterol R CH3 Ph CH Tertiary alcohol OH CH3-C-OH Ph一C一OH OH CH3 Ph 2-methyl-2-propanol triphenylmethanol 1-methylcyclopentanol OH OH OH Phenols OH CH3 HO phenol 3-methylphenol hydroquinone
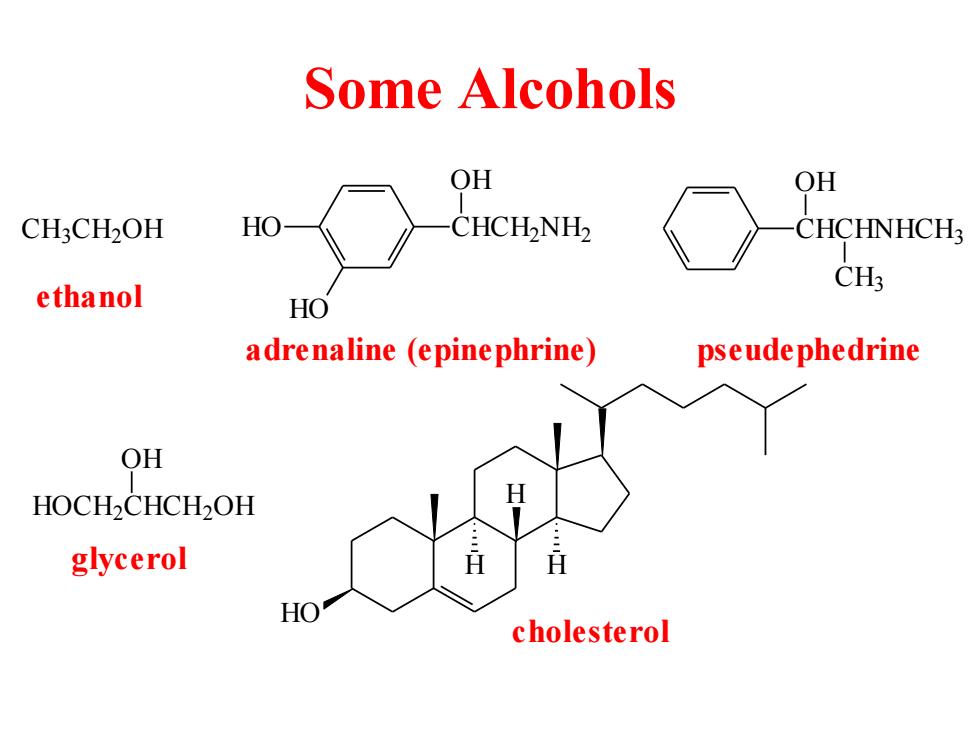
Some Alcohols OH OH CH:CH2OH HO CHCH-NH2 CHCHNHCH3 CH; ethanol HO adrenaline (epinephrine) pseude phedrine QH HOCH2CHCH2OH glycerol HO cholesterol
Some Alcohols C H3C H2O H HO HO CHCH2NH2 OH adrenaline (epinephrine) ethanol CHCHNHCH3 OH CH3 pseudephedrine HO H H H cholesterol HOCH2CHCH2O H OH glycerol
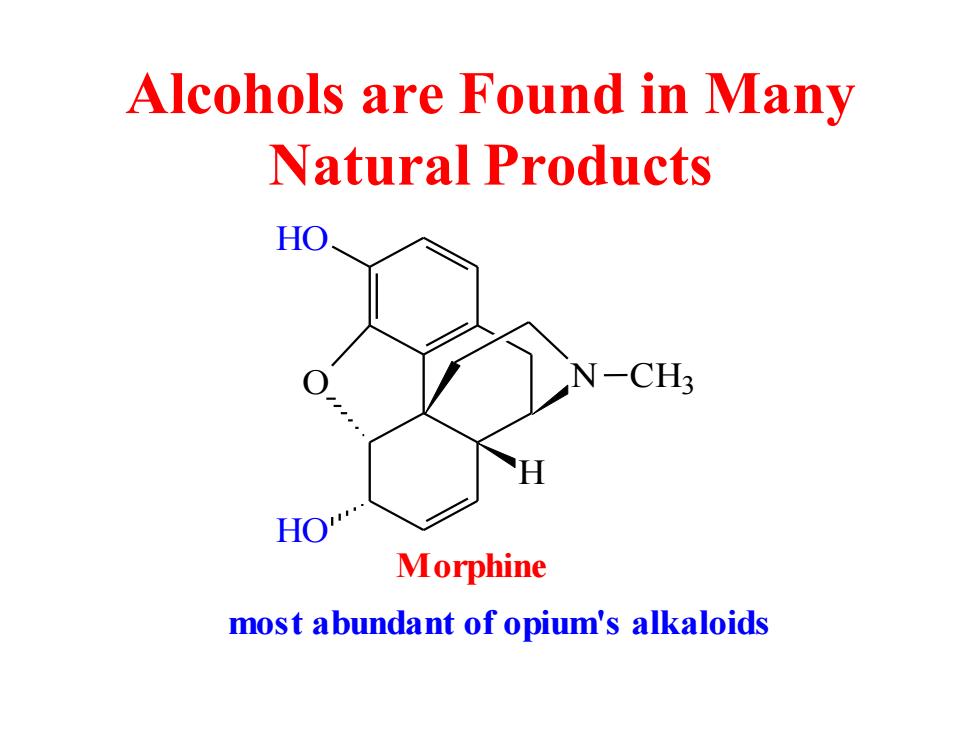
Alcohols are Found in Many Natural Products HO N-CH3 HO Morphine most abundant of opium's alkaloids
Alcohols are Found in Many Natural Products O HO HO N CH3 H Morphine most abundant of opium's alkaloids
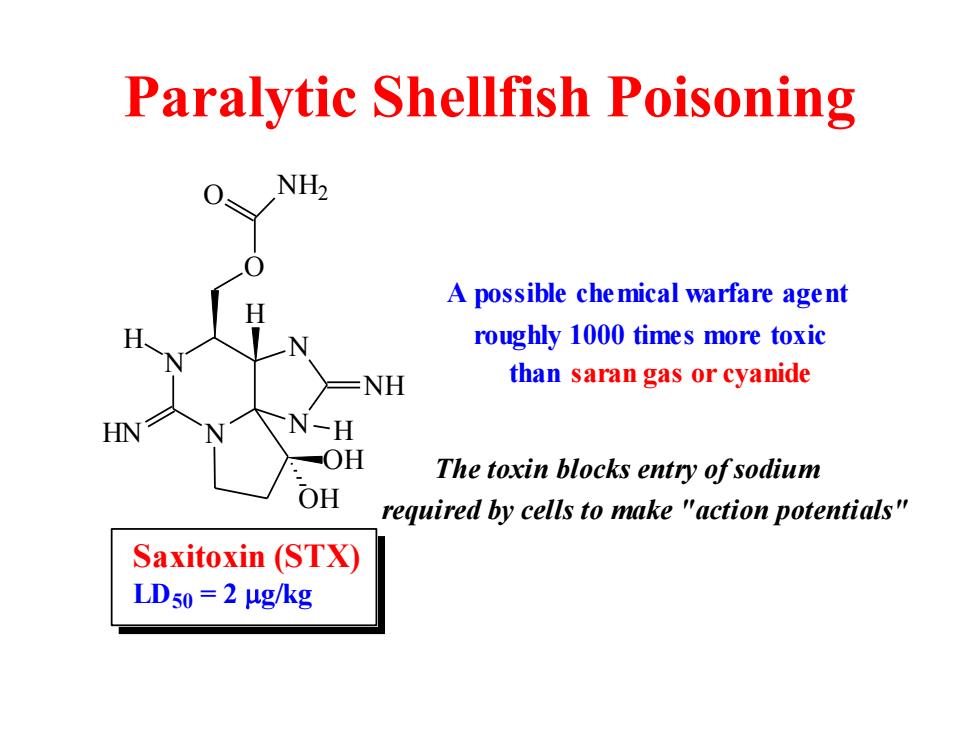
Paralytic Shellfish Poisoning NH2 A possible che mical warfare agent roughly 1000 times more toxic -NH than saran gas or cyanide HN N-H -OH The toxin blocks entry ofsodium OH required by cells to make "action potentials" Saxitoxin (STX) LD50=2μg/kg
Paralytic Shellfish Poisoning N N N N OH OH H HN H O O NH2 NH H Saxitoxin (STX) LD50 = 2 g/kg A possible chemical warfare agent roughly 1000 times more toxic than saran gas or cyanide The toxin blocks entry of sodium required by cells to make "action potentials
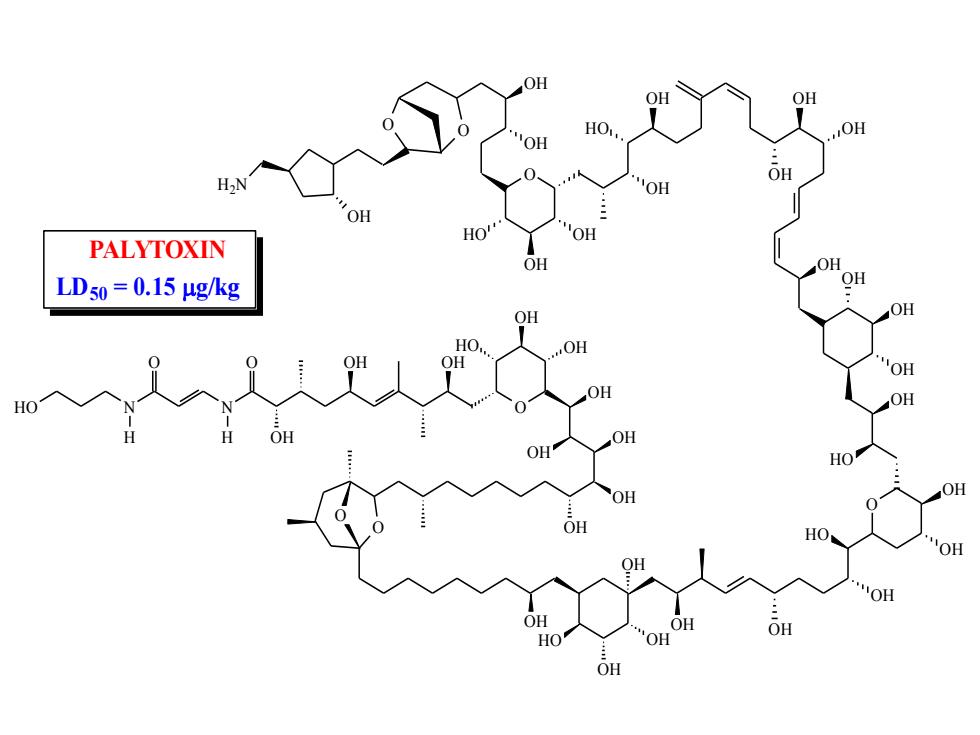
OH OH HO OH OH H2N OH OH OH HO OH PALYTOXIN OH OH LDs0=0.15μg/kg OH OH OH "OH HO OH OH OH HO OH OH OH HO OH OH OH OH HO OH
HO N N H O H O OH OH OH O HO OH OH OH OH OH OH O OH O OH OH OH HO OH OH OH OH HO O OH OH OH HO OH OH OH OH OH OH OH OH OH HO O OH OH HO OH OH O O OH H2N PALYTOXIN LD50 = 0.15 g/kg
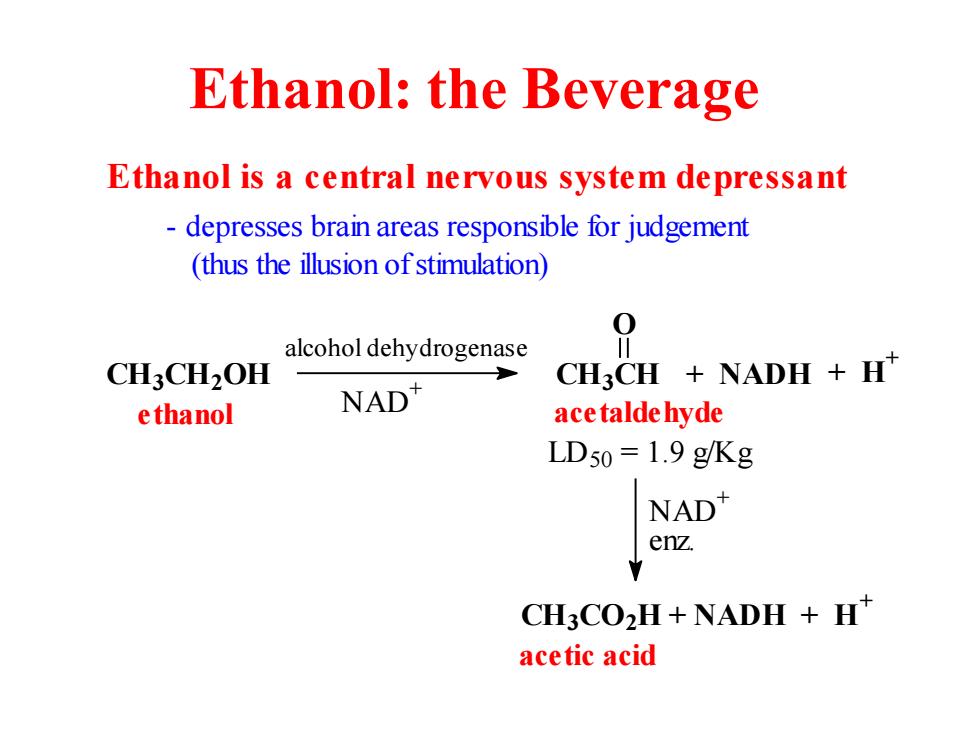
Ethanol:the Beverage Ethanol is a central nervous system depressant depresses brain areas responsible for judgement (thus the illusion of stimulation) 0 alcohol dehydrogenase CH3CH2OH CH3CH NADH H" e thanol NAD" ace talde hyde LD50=1.9 g/Kg NAD enz. CH3CO2H+NADH +H acetic acid
Ethanol: the Beverage enz. CH3CO2H + NADH + H+ NAD+ CH3CH2OH CH3CH O acetaldehyde LD50 = 1.9 g/Kg ethanol Ethanol is a central nervous system depressant - depresses brain areas responsible for judgement (thus the illusion of stimulation) alcohol dehydrogenase NAD+ + NADH + H+ acetic acid
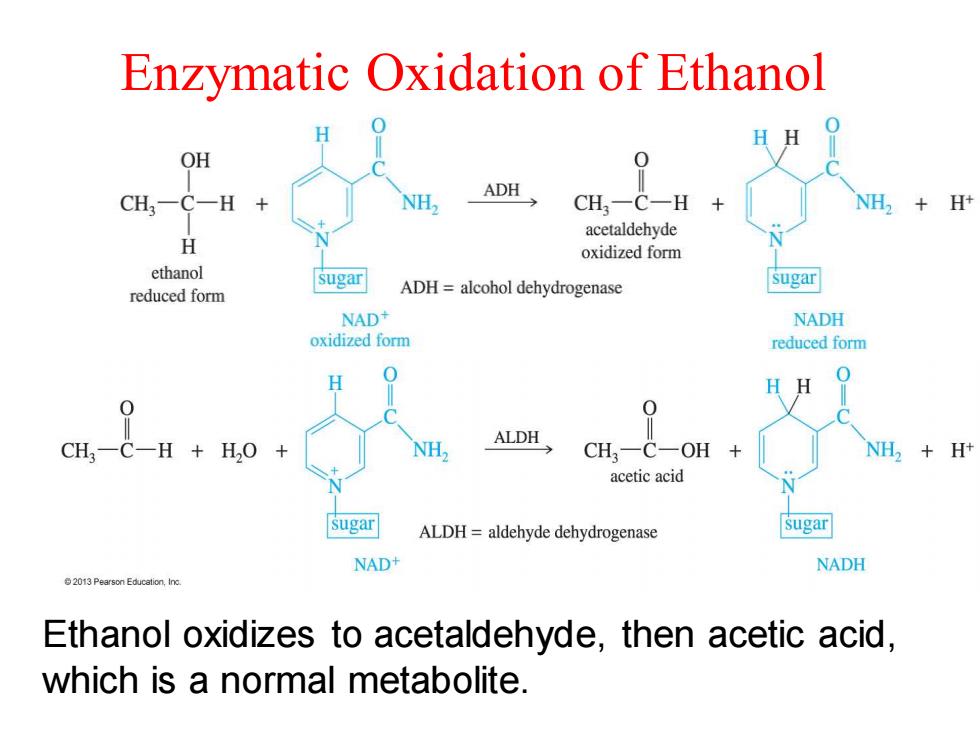
Enzymatic Oxidation of Ethanol HH OH ADH CH3一 CH,一C一H NH,H+ acetaldehyde H oxidized form ethanol sugar sugar reduced form ADH=alcohol dehydrogenase NAD+ NADH oxidized form reduced form 0 HH CH-C-H +HO ALDH NH CH-C-OH NH,H+ acetic acid sugar ALDH=aldehyde dehydrogenase sugar NAD NADH Ethanol oxidizes to acetaldehyde,then acetic acid, which is a normal metabolite
Enzymatic Oxidation of Ethanol Ethanol oxidizes to acetaldehyde, then acetic acid, which is a normal metabolite