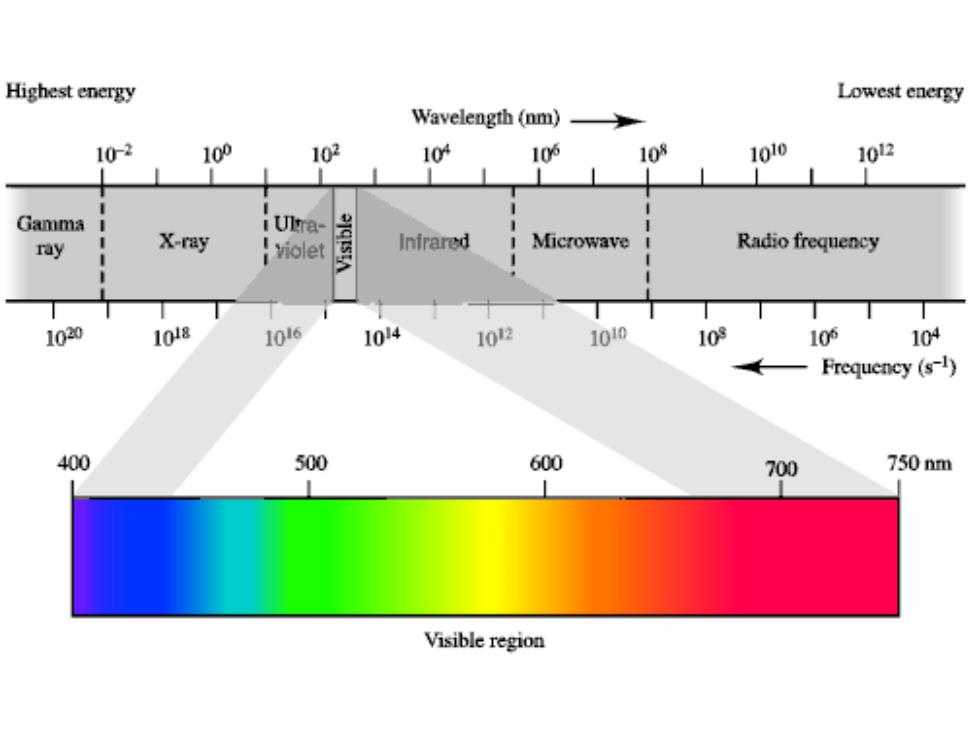
Highest energy Lowest energy Wavelength (nm) 10-2 10P 102 10 10的 108 1010 102 Gamma i Ulra- ray X-ray iviolet Inirared Microwave Radio frequency 1020 1018 106 104 102 1010 109 10的 104 Frequency (s-1) 400 500 600 700 750nm Visible region
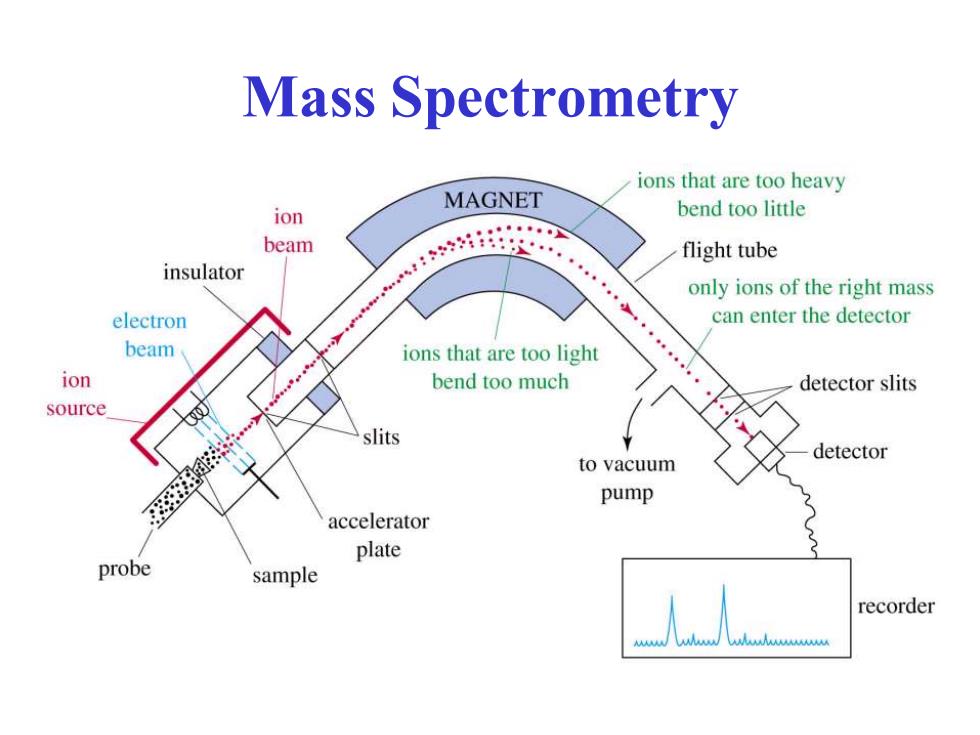
Mass Spectrometry ions that are too heavy MAGNET ion bend too little beam flight tube insulator only ions of the right mass electron can enter the detector beam ions that are too light ion bend too much detector slits source slits detector to vacuum pump accelerator plate probe sample recorder
Mass Spectrometry
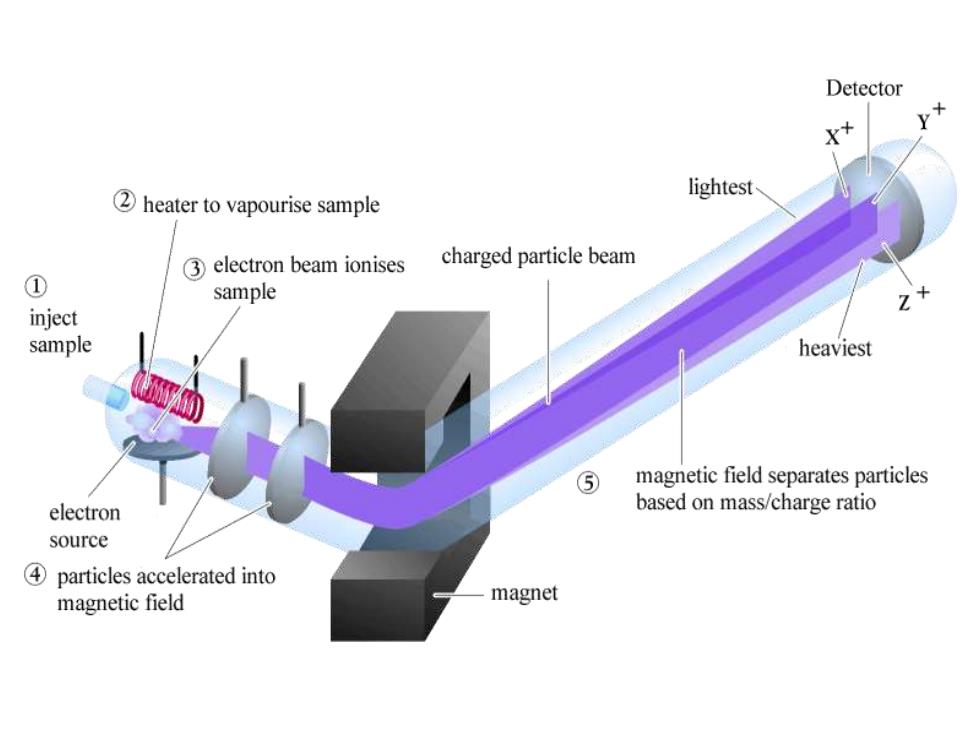
Detector lightest 2 heater to vapourise sample 3 electron beam ionises charged particle beam ① sample Z inject sample heaviest ⑤ magnetic field separates particles electron based on mass/charge ratio source 4 particles accelerated into magnetic field magnet
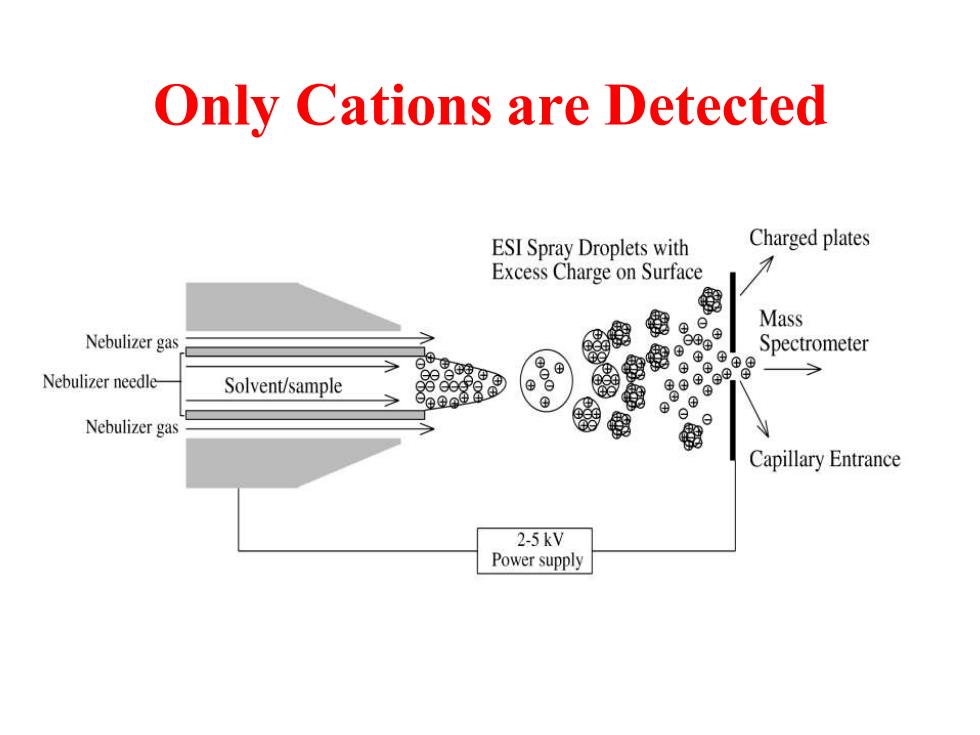
Only Cations are Detected ESI Spray Droplets with Charged plates Excess Charge on Surface 倒 Mass Nebulizer gas ⊕ Spectrometer ⊕© Nebulizer needle- Solvent/sample 8o88 Nebulizer gas 我 Capillary Entrance 2-5kV Power supply
Only Cations are Detected
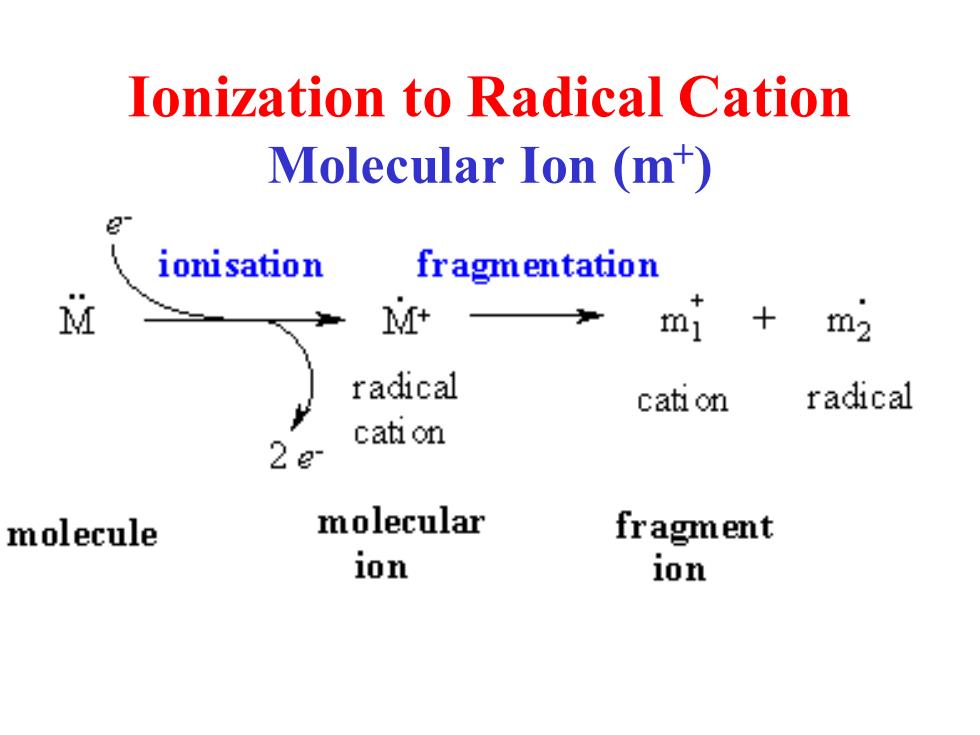
lonization to Radical Cation Molecular Ion (m) ionisation fragmentation M M+ mI m2 radical cati on radical cati on 22 molecule molecular fragment ion ion
Ionization to Radical Cation Molecular Ion (m+ )

Glossary Molecular ion -The ion obtained by the loss of one electron from the molecule (m) Base peak-The most intense peak in the MS, assigned 100%intensity Radical cation-positively charged species with an odd number of electrons Fragment ions-Lighter cations (and radical cations)formed by the decomposition of the molecular ion.These often correspond to stable carbcations. .m/z-mass to charge ratio
Glossary • Molecular ion - The ion obtained by the loss of one electron from the molecule (m+ ) • Base peak - The most intense peak in the MS, assigned 100% intensity • Radical cation - positively charged species with an odd number of electrons • Fragment ions - Lighter cations (and radical cations) formed by the decomposition of the molecular ion. These often correspond to stable carbcations. • m/z - mass to charge ratio
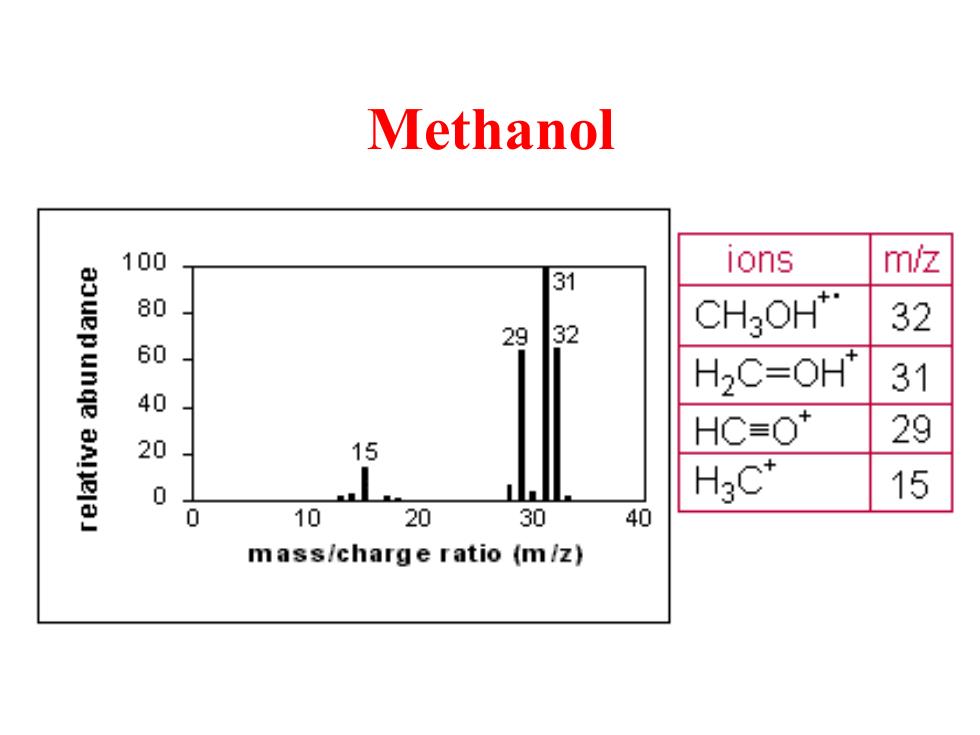
Methanol 100 ions m/z 31 aouepunge 80 29 32 CH3OH* 32 60 H2C=0H* 31 40 20 HC≡O 29 15 0 H3C* 15 0 10 20 30 40 mass/charge ratio (m/z)
Methanol
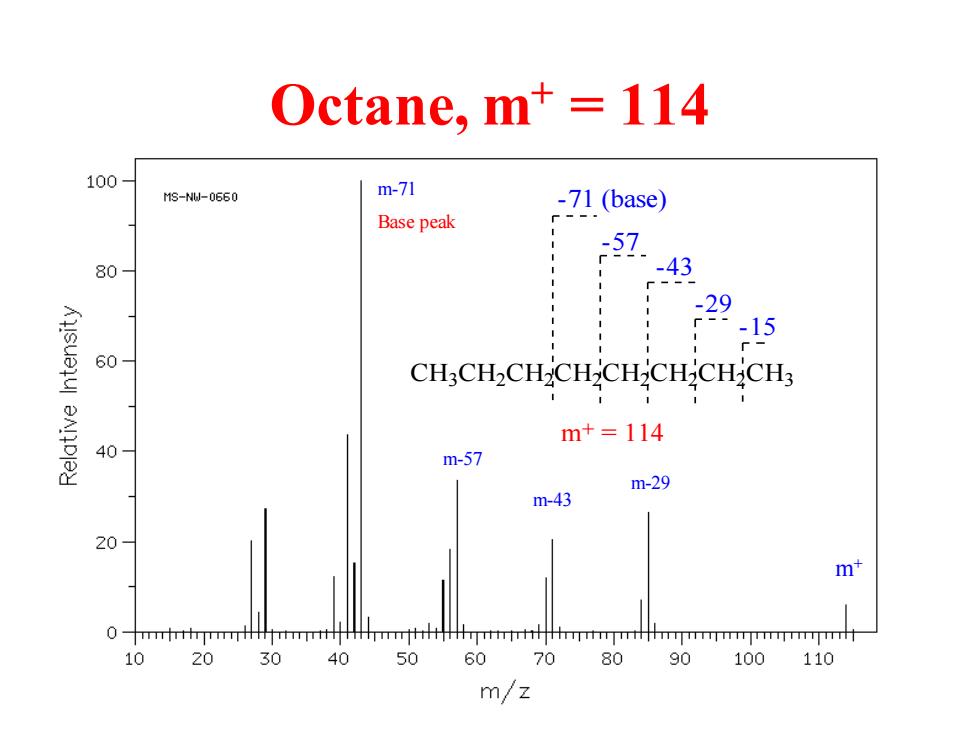
Octane,m+114 100 1s-N1-0560 m-71 -71(base) Base peak - 57 80 -43 -29 -15 60 CH:CH2CH-CH-CH-CH-CH2CH3 m+=114 40 m-57 m-29 m-43 20 m+ 0 -rrmmmllmrHrmhhpmtmmhmmmmpmr 10 20 30 40 50 60 70 80 90 100 110 m/z
Octane, m+ = 114 CH3CH2CH2CH2CH2CH2CH2CH3 m+ = 114 -15 -29 -43 -57 -71 (base) m-29 m-43 m-57 m-71 Base peak m+
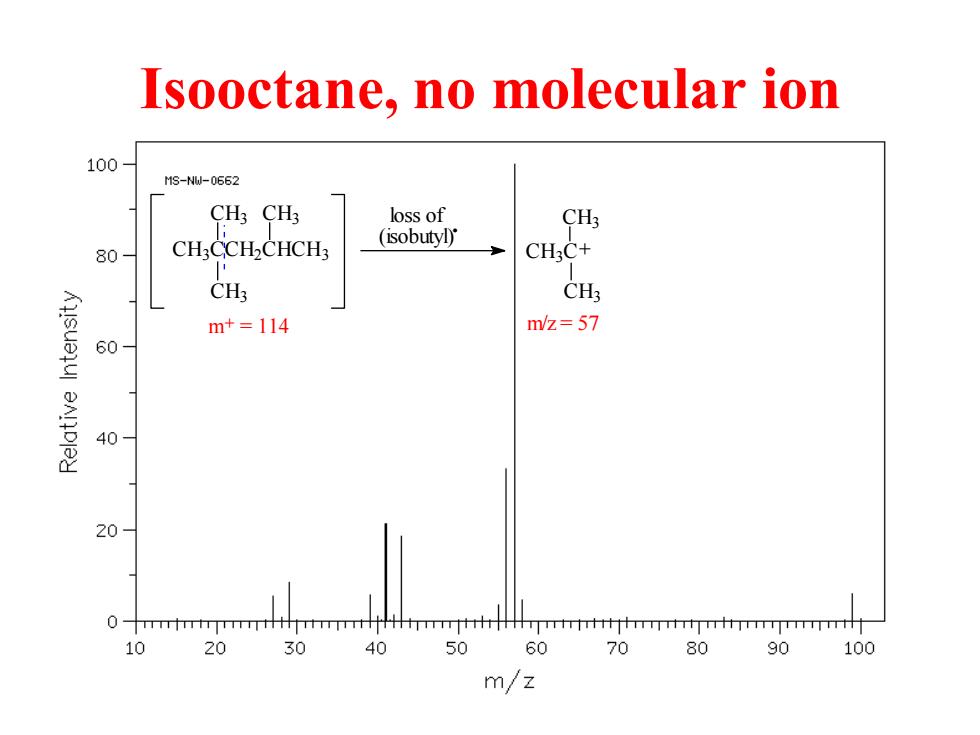
Isooctane,no molecular ion 100 M3-NW-0662 CH3CH3 loss of CH3 CH:CCH2CHCH3 (isobutyl) 80 CH3C+ CHs CH3 m+=114 m/z=57 60 BAlDley 40 20 10 20 30 40 50 60 70 80 90 100 m/z
Isooctane, no molecular ion CH3CCH2CHCH3 CH3 CH3 CH3 m+ = 114 loss of (isobutyl). CH3C CH3 CH3 + m/z = 57
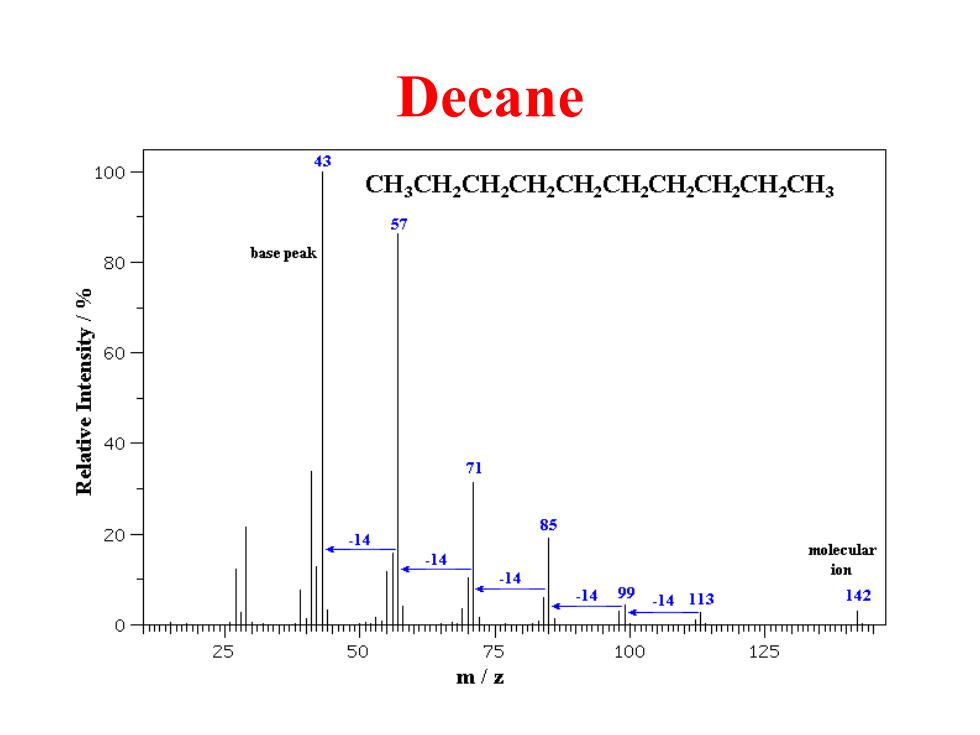
Decane 43 100- CH.CH.CH.CH.CH.CH.CH.CH.CHCH 57 80 base peak 3 HSUu 60 SAREPH 40 71 85 20 -14 -14 molecular -14 ion 149914113 142 0-mmmtmirmmmmimmtnmmmhmmmjrmmmnmmmmjmmmmmmmim 25 50 75 100 125 m/z
Decane